Pyrolysis is one of the thermal treatments for biomass wastes that results in the production of liquid, solid and gaseous products. Unfortunately, the complex structure of the biomass materials matrix needs elevated heating to convert these materials into useful products. Microwave heating is a promising alternative to conventional heating approaches. Recently, it has been widely used in pyrolysis due to easy operation and its high heating rate. This review tries to identify the microwave-assisted pyrolysis treatment process fundamentals and discusses various key operating parameters which have an effect on product yield. It was found that several operating parameters govern this process such as microwave power and the degree of temperature, microwave absorber addition and its concentration, initial moisture content, initial sweep gas flow rate/residence time. Moreover, this study highlighted the most attractive products of the microwave pyrolysis process. These products include synthesis gas, bio-char, and bio-oil.
1. Introduction
Biomass waste is a biopolymer abundant in nature as dry plant matter and as a low-value by-product of different activities by various industrial sectors such as forestry and argo-industrial residues (e.g., straws, husks, wood, trunks, peel, and bark), municipal solid waste (e.g., kitchen waste, waste paper and cardboard, wood items, and garden residues) and the effluents of wastewater treatment plants (e.g., sludge) [1,2]. The accumulation of this poses problems, safety hazards and health issues, as well as impacting on sustainable development in terms of the recycling of waste materials and resource recovery [3,4,5]. Since biomass wastes are biodegradable, they represent a significant source of renewable organic matter [6]. These features of biomass waste have encouraged research throughout the world to utilize these materials in sustainable technology development to solve such problems, i.e., to reduce waste and to generate clean and renewable energy at the same time.
Several conversion processes such as physical, thermal, biochemical–microbial and chemical have been utilized to transform biomass into energy [7,8]. It is important to note that the treatment of wastes is essential due to the restrictions in disposal, which are mainly related to cost and space. Incineration is the thermal treatment typically used in thermal waste treatment. Nonetheless, it leads to several problems such as air pollution (e.g., dioxins and furans), high cost, and ash disposal, to name a few [9]. In view of cost and space problems, reduction of volume has been the target of waste treatment. Pyrolysis has been proposed to achieve volume reduction and generate valuable products (bio-fuel) instead of incineration which is not viable. Air emissions usually pose the key environmental issue for most combustion systems, and due to the high cost of strict pollution control or pollution mitigation or compensation measurements. They have been a major obstacle to the viability of biomass combustion in many low air quality regions. Comparatively, the biomass pyrolysis products provide options for pollution relief for greenhouse gas and practical options for the particulate emissions of biomass combustion such as producing heat and electricity. Biomass pyrolysis thermally transforms biomass feedstock into bio-oil, biochar, and syngas in the absence of air/oxygen [10]. In this process, the organic material is thermally degraded by cracking the chemical bonds in an inert environment [11]. Generally, the end product of pyrolysis process are energy recovery products which have a high energy efficiency and generate minimal atmospheric emissions, etc. These products include gaseous, solid and liquid products (i.e., syngas, bio-oil, and bio-char). For many years, the traditional pyrolysis of biomass has been implemented by an electrical furnace and continually purged with nitrogen [12]. Due to microwave heating advantages against traditional heating, the successful implementation of microwave heating for thermal biomass waste treatment [13,14,15] has encouraged extensive research into microwave-assisted pyrolysis of biomass waste in the last decade. Current studies in microwave technologies seem to be offering the best waste management solution, allowing a range of microwave systems to be designed, developed, and optimized to process various waste products. It has been widely employed for biomass chemical transformation into valuable products [16,17]. Microwave energy technologies may deliver: i) a reduction in waste volume, (ii) selective heating, (iii) rapid heating, (iv) increased capability for treating waste in-situ, (v) chemical reactivity, (vi) rapid and flexible processes that can also be remotely controlled, (viii) ease of control, (viii) energy savings, (ix) overall cost effectiveness, (x) equipment portability, and (xi) compared to some more traditional systems, cleaner energy sources, etc. [18].
The earliest known pyrolysis plant, utilizing microwave energy to breakdown polymers in used tires, was developed in the UK in 1989 [19]. Since the 1990s, the patents for the microwave pyrolysis of wastes have become abundantly available in the United States. In the last few years, many attempts have been made regarding microwaves to turn them into a possible route for biomass pyrolysis process treatment and converting biomasses into liquids and solids [20,21,22,23]. Various types of solid waste and biomass feedstock were recently utilized in microwave pyrolysis processes such as oil palm shell [24], paper deinking residue [25], plastics [26], wood sawdust [27], oily sludge [28], sewage sludge [29], cellulose [30], gumwood [31], and rice straw [32]. There are several challenges to be overcome for the processing of various materials applying microwave energy. The problems related with microwave processing of waste materials include the inherent difficulties with microwaves itself and another one inherent with the processed materials [33]. These difficulties include some aspects such as the ability of a material to change microwave energy to heat depending on the dielectric properties. Dielectric heating is a volumetric procedure whereby the heat is generated inside the material via selective absorption of electromagnetic energy. However, not all materials (e.g., transparent material) are easily heated by microwave heating. For instance, the materials are of high moisture whereby microwave heating can be very energy efficient compared to dry materials. Moreover, adding absorbers to transparent materials could assist the rise in reaction temperature. Another aspect is the challenge of measuring temperature in the microwave environment and the non-uniform heating behavior of the microwave is responsible for thermal damage in processed materials. This necessitates the need of special instrument design because the uniformity of the microwave field can be enhanced by increasing the size of the cavity. Moreover, the microwave leakage may harm the human being, requiring health and safety precautions and careful processing procedures.
2. Microwave-Assisted Pyrolysis
Generally, different conditions of microwave-assisted pyrolysis will lead to varying yields of products. The efficacy of microwave pyrolysis ultimately relies on the operating conditions of this process. The factors that have the most impact on product recovery in microwave pyrolysis are temperature rate, microwave absorber addition, and its concentration (e.g., metal oxides and sulfides, carbon based materials and silicon carbide), initial moisture content, and initial sweep gas flow rate/residence time. It is essential to understand the effects and interactions between these parameters during the microwave assisted pyrolysis process. So, this section will highlight some of the facts and guidelines regarding the selection of operational conditions and the interactions between parameters. For instance, biomass pyrolysis at a low temperature, with a high heating rate and a short gas residence time, will favor the yield of liquid products. Meanwhile, low temperature and a low heating rate condition are required for maximum char production. However, high gas production will result from a high temperature, with a low heating rate and long gas residence time process.
2.1. Effect of Feedstock Characteristics
The microwave-assisted pyrolysis of various biomass feedstocks shows that biomass waste type (i.e., sludge wastes, agro-industry residues, forest biomass) and the feedstock characteristics (i.e., moisture (wt%), the components of proximate analysis (wt%), the elements of ultimate analysis (wt%), the fractions of lignocellulosic analysis, higher (or gross) heating value (HHV) and low (or net) heating value (LHV), and the particle size) directly influence the final products of pyrolysis process at different operating conditions [
62]. For instance, the microwave-assisted pyrolysis of corn stover, bamboo leaves, rice husk, rice straw, sugarcane peel, sugarcane bagasse, and waste coffee grounds was achieved using similar treatment conditions [
63]. This study showed there is no significant differences in the solid yields of the seven feedstocks. On the contrary, the liquid, and gas yields are highly influenced based on the type of feedstock. It was found that the corn stover had the lowest liquid yield, but it had the highest gas yield (40 wt%). Meanwhile, rice husk had the highest yield of liquid (48 wt%) but it had the lowest yield of gas (30 wt%).
In the recent decades, waste sludge has been widely utilized in the global bioenergy or biogas production sector. The recent research has recorded that the microwave-assisted pyrolysis treatment offers certain advantages over other approaches in managing sewage sludge wastes [
64]. Earlier, Menendez et al. [
65] reported that wet sewage sludge could not be heated at temperatures higher than 200 °C unless mixed with a suitable microwave absorber. So, carbonaceous char residues from the previous experiments were used as an absorber. Dominguez et al. [
66] conducted research to determine the feasibility of microwave and conventional pyrolysis for the production of synthesis gas (syngas) from sewage sludge. Two kinds of sludge were applied: aerobic and digested anaerobic sludge. When wet sewage sludge was pyrolyzed using microwave heating, the effect of the moisture content in producing higher quantities of H
2 and CO was observed. Nevertheless, wet sewage sludge produced higher hydrocarbons from conventional pyrolysis resulting in a greater HHV for gases produced from conventional heating (15–17 MJ/m
3) compared to the gas produced from microwave heating (13 MJ/m
3).
The sewage sludge was pyrolyzed under microwave heating at temperatures up to 1000 °C using graphite as a microwave absorber [
67,
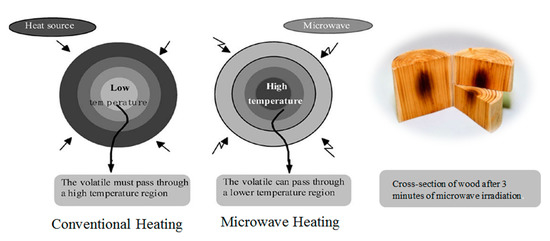
68]. A conventional electric furnace also was utilized to pyrolyze the sample to compare the outcomes gained from both approaches. The sample was homogenously blended with the graphite, and then heated for about 6 min. The bio-oil products obtained from the microwave pyrolysis have a high calorific value (33.9–36.8 MJ/kg) and a low proportion of polycyclic aromatic hydrocarbons (PAHs) compounds. On the other hand, the bio-oil produced from traditional heating at high temperature enriched the PAH content. The use of microwave instead of using conventional heating was expected to be advantageous to pyrolyze large pieces of wood, thus perhaps eliminating the need to cut the sample [
69]. During conventional heating, the materials are heated thoroughly as the heat is transmitted via conduction and/or convection from the surface into inside of heated matter. In contrast, microwave heating might induce hotspots within the material; therefore, the heating could be generated within the heated matter without the need for a transmission distance [
70] as illustrated in .
Figure 3. Heat transfer in the conventional and microwave heating of wood [
70]. (Reproduced with permission from Elsevier)
.
A sewage sludge pyrolysis under conventional and microwave was carried out to evaluate the feasibility of producing syngas [
71]. The total concentration of syngas was higher under microwave heating with values up to 85 vol% while only 74 vol% was for conventional heating. However, the calorific value of the gaseous products also increased from 17–25 MJ/kg in microwave pyrolysis compared to 17–20 MJ/kg in conventional pyrolysis as a result of the high hydrocarbon concentration in the gas from conventional pyrolysis. Mamaeva et al. [
72] pyrolyzed pine sawdust using microwave heating to produce phenolic rich bio-oil. They showed that the maximum content of the phenolic compounds reached 61.19% (area) at 300 °C. On the other hand, another research study was carried out by Chen et al. [
73] where pine wood sawdust was pyrolyzed at 470 °C. They found that the yield of liquid products depended on the type of additive. For instance, the relatively high yield of liquid product resulted from the sample mixed with H
3PO
4. This might be related to the transfer of a considerable part of the added H
3PO
4 to the liquid because the boiling point of H
3PO
4 is slightly higher than 200 °C. The main components of the gaseous products were CO, CH
4, CO
2 and H
2. Meanwhile, performing the analysis of the liquid products using a gas chromatograph with a thermal conductivity detector (TCD) showed that there were six organic compounds in the liquid fraction, which were acetol, 2-furanmethanol, furfural, guaiacol, 4-methyl-guaiacol, and levoglucosan. Comparing with the pyrolysis of wood using conventional heating in other studies, a higher distribution of organics was found. Microwave heating may have increased the secondary reaction of levoglucosan produced as gas due to the higher actual temperature of the liquid products governed by dielectric heating. These outcomes confirm that the internal heating mechanism leads to rapid heating, which is more effective than traditional electric heating systems [
74]. The bio-oil gained from strong microwave-assisted waste pyrolysis can be further utilized as alternatives or mixtures for petroleum and diesel with better characteristics of high H content, low C content, rich monoaromatic compounds, etc. [
66,
75,
76].
2.2. Effect of Temperature Rate
In general, microwave heating is expressed in terms of the microwave power output that can be set on the microwave reactor. Normally the temperature in the reactor during the heating treatment is measured using thermocouples. To date, many experimental researches have been implemented to investigate the temperature effect on the pyrolysis of biomass [
77,
78]. Inguanzo et al. [
79] documented that the increase in the pyrolysis temperature of sewage sludge led to a decrease in the solid fraction. However, an increase in the pyrolysis temperature caused an increase the gas fraction productivity meanwhile the liquid products remained almost constant. This can be associated to the mechanism of the pyrolysis process, whereby higher temperature promotes the secondary cracking reactions and gives a higher gas yield. It was reported that pyrolysis of pomegranate seeds at temperatures between 400 to 800 °C resulted in a significant amount of the produced liquid at the temperature of 500 °C [
80]. However, the pyrolysis temperature did not affect the compositions of bio-oil. Interestingly, the amount of the produced gas, especially CH
4 and CO, increased when the pyrolysis temperature was increased. A similar trend also was reported when grape bagasse was pyrolyzed at temperatures of 350 °C to 600 °C [
81]. As the temperature elevated from 350 °C to 600 °C at a heating rate of 10 °C/min, the maximum gas yields were obtained at a value of 39.4%. Furthermore, at a higher temperature up to 900 °C, pore volumes and surface area of bio-char gained from fast pyrolysis of maize stalk chars were significantly enhanced and reached the maximum value at 0.038 cm
3/g, 0.029 cm
3/g and 81.63 m
2/g for the micropore, mesopore and surface area, respectively [
82]. Nonetheless, at a temperature over 900 °C, the pore volume and surface area slightly decreased which was caused by predomination of structural ordering, widening pores and coalescence of neighboring pores. This might also result from the softening; melting, fusing and carbonization caused pores in the bio-chars to be partially blocked. Therefore, this would inhibit the adsorption of gas to the pores and lead to a lower surface area.
2.3. Effect of Microwave Absorber Addition and Concentration
Biomass is regularly a low microwave absorber. However, adding the microwave absorbing substances enhances the microwave absorption capacity of treated materials. Many research studies have been conducted. They examined the process of microwave-assisted pyrolysis of biomass without microwave absorbers, and with additives of inorganic substances as absorbers. It was found that utilizing the microwave absorbers can lead to enhancement of the pyrolysis reaction temperature at relatively low microwave power. The microwave absorbers can increase the temperature of the reaction by indirectly heating the surrounding particles of biomass, which finally affect the yield and quality of the product. By the same method, some of studies showed that the microwave absorbers and some catalysts the to the biomass can adjust the distribution of products, improve the energy efficiency of the process or enhance the content of specific bio-oil, bio-char, and gas components under various treatment conditions [
83,
84,
85,
86,
87,
88,
89]. For instance, the effectiveness of microwave absorber addition and concentration for empty fruit bunch pyrolysis was studied by Omar et al. [
90]. The samples were added with 5% char obtained from the previous pyrolysis. A higher maximum temperature was obtained at 590 °C compared to that without the absorber of only 177 °C. Increasing the char to 10 and 15% did not seem able to elevate the operating temperature. However, the increase in the char concentration enhanced the yield of hydrogen gas while at the same time reducing the yield of CO and lowering the heating value (LHV). The microwave absorber effect on the microwave pyrolysis of oil palm biomass was investigated by adding oil palm shell char that resulted from traditional pyrolysis [
91]. The absorber size was in the range of 100 to 300 μm. The addition of the microwave absorber at a ratio of 1:0.25 biomass to microwave absorber gives a maximum yield of bio-char at the value of ~70%. This suggested that biomass could not be heated sufficiently when an only small amount of microwave absorber was added to the biomass. Conversely, bio-oil and gas products were produced at a highest ratio of 1:0.5 biomass to microwave absorber at values of ~25 and 30%, respectively. At this ratio, the attainable maximum operating temperature was at 273 °C.
The combination of char with sewage sludge pyrolyzed under microwave heating improved the syngas production up to 66% compared to that mixed with graphite produced a lower improvement of 62% [
92]. These values were much higher than those produced by traditional pyrolysis. The heating values of the gases from microwave pyrolysis ranging from 7 to 9.5 MJ/m
3 were lower than that obtained from conventional pyrolysis (~14 MJ/m
3).
Five metal oxides including, Al
2O
3, CaO, Fe
2O
3, TiO
2 and ZnO were added into demineralized sewage sludge to be heated under microwave pyrolysis [
93]. The addition of Fe
2O
3 and ZnO found was found to favor the formation of solid residue while on the other side it was reduced by adding Al
2O
3, CaO and TiO
2. Hence, it can be assumed that the existence of Al
2O
3, CaO and TiO
2 encouraged the degradation of organics matters, causing only small amount of residue to be obtained. Furthermore, the addition of CaO may support the initial hemicellulose decomposition and further lignin degradation when it enhanced the sludge conversion temperatures below 177 °C and above 487 °C. At temperatures above 237 °C, Fe
2O
3 restrained the decomposition of cellulose and lignin since it was observed that Fe
2O
3 only enhanced the conversion of sludge at low temperatures.
2.4. Effect of Initial Moisture Content
Biomass wastes typically have a low ability for microwave absorption. However, the existence of relatively high moisture and inorganic substances may enhance the end product. It was reported that products yields (i.e., bio-oil, bio-gas, and bio-char) gained from microwave-assisted pyrolysis strongly depend on the initial moisture content of the involved feedstock [
22]. Three different levels of the initial moisture content (4, 40 and 75%) were applied during the pyrolysis of oily sludge in a microwave environment [
28]. The highest volume reduction was achieved in samples with 75% moisture content, samples whereby only a small amount of solid product was left after the pyrolysis as a result of the increase in temperature evolution in biomass samples for the samples of high moisture content. Moreover, it was observed that the amount of liquid products was directly proportional to the moisture content of each sample. The influence of the initial moisture content on the yield of liquids from the pyrolysis of sawdust was studied in a stainless-steel tube (95 mm height × 17 mm inner diameter) [
94]. The yields of liquid products increased with the increase in the initial moisture content of the sample. The presence of water in the biomass was found to affect the physical properties and quality of the resultant liquid products. In another research utilizing the same reactor, traditional pyrolysis of spruce wood, hazelnut shells and wheat straw was carried out. The objective was to study the effects of the initial moisture content on the oily products yield and their higher heating values [
35]. The drier feedstock was found to encourage the production of high viscosity oil, especially at the higher reaction temperature. The results reported that for higher initial moisture content, the maximum liquid yield on a dry feed basis occurred at lower pyrolysis temperatures of between 416 °C and 430 °C.
2.5. Effect of Initial Sweep Gas Flow Rate/Residence Time
The residence time of a volatile phase is variable depending on the flow rate of the carrier gas, such as nitrogen and helium [
95,
96]. It is used to remove the volatiles from the pyrolysis environment. Minimum secondary reactions such as thermal cracking, repolymerization and re-condensation are very important to obtain maximum liquid yield. The higher gas flow rate will shorten the residence time in the reactor [
97]. Thus, their chance to be involved in char forming via secondary decomposition of higher molecular-weight products can be reduced [
98]. Various sweep gas flow rates of Nitrogen (200, 400 and 600 mL/min) were applied to pyrolyze Oily sludge under microwave heating to identify the optimum feeding rate and explore the effect of inert gas on the operating temperature condition and product distribution [
28]. The temperature profile of the flow rates of 400 and 600 mL/min took approximately 10 min or purpose drying at a temperature around 100 °C before the temperature increased to around 300 °C at the 20th min until the end of the process. On the contrary, applying a flow rate of 200 mL/min resulted in a slightly higher temperature of 321 °C that was achieved after 20 min. Therefore, Mokhtar and his co-workers [
28] concluded that the nitrogen flow rate of 200 mL/min was the optimum feeding rate for microwave-assisted pyrolysis of oily sludge. Fast pyrolysis of sugarcane bagasse was achieved by Parihar et al. [
98]. The oil and char yields decreased from 22 to 19% and 27 to 21%, respectively, as the N
2 flow rate increased (from 50 to 200 cm
3 min
−1) while the gas yield increased linearly from 14 to 23%. The bio-oil obtained from this experiment contains only 0.09 wt% sulfur, so very low emissions of SOx are expected since the ash content also is very low (0.02 wt%). The energy content of the bio-oil (37 MJ kg
−1) is approximately equal to coal (32–37 MJ kg
−1) and lower compared to petroleum fuels such as that used in a gas turbine (about 42 MJ kg
−1).The influence of residence time on sawdust and rice straw pyrolysis was studied by Chen et al. [
99]. The residence time required for the optimum pyrolysis was noticeably longer compared to that reported in the literature. This could be due to the low average temperature and low reactivity of the biomass fuel. A significant increment of gas yield was observed in the pyrolysis of sawdust and rice straw at a 2.0 and 3.0 s residence time. Mastral et al. [
100] reported that the residence time gives an apparent effect on the product distribution and gas composition especially when the temperature increased from temperatures of 645 °C to 780 °C during the pyrolysis of high-density poly-ethylene (HDPE). It could be noticed that the yield of the gas production increased from 11.4% at 1 s to 31.5% at 1.5 s due to the secondary decomposition reaction. As the temperature rose to up to 780 °C at 1.3 s, a higher gas yield was obtained at a value of 86.4% while the rest were oil and waxes.
Bibliogarghy
Ethaib, S.; Omar, R.; Kamal, S.M.M.; Awang Biak, D.R.; Zubaidi, S.L. Microwave-Assisted Pyrolysis of Biomass Waste: A Mini Review. Processes 2020, 8, 1190. https://doi.org/10.3390/pr8091190
This entry is adapted from the peer-reviewed paper 10.3390/pr8091190