Benign prostatic hyperplasia (BPH) is a proliferative disorder of the prostate gland arising from its epithelial cells and smooth muscle within the transitional zone. Prostatitis is a common urologic condition that in 1999 was subdivided into four categories—acute bacterial prostatitis, chronic bacterial prostatitis, chronic non-bacterial prostatitis/chronic pelvic pain syndrome (CPPS), and asymptomatic inflammatory prostatitis. Urinary tract infection (UTI) is one of the most common bacterial infections affecting women. Benign prostatic hyperplasia, urolithiasis, recurrent urinary tract infections, and chronic prostatitis are diseases that are commonly diagnosed worldwide. Carotenoids, including lycopene, are widely available in fruits and vegetables, and it is postulated that they can be used in the prevention and treatment of benign urological conditions.
- urinary tract infections
- benign prostate hyperplasia
- lycopene
1. Benign Prostatic Hyperplasia
1.1. Benign Prostatic Hyperplasia Epidemiological Studies
1.2. Benign Prostatic Hyperplasia Experimental Studies
2. Prostatitis
2.1. Prostatitis Experimental Studies
2.2. Prostatitis Clinical Studies
| Study | Year | Studied Population | Intervention | Results | Ref. |
|---|---|---|---|---|---|
| Cai et al. | 2016 | 79 patients suffering from CBP | The participants were assigned to one of two groups: Group A taking levofloxacin 500 mg once daily for two weeks with lycopene and methylsulfonylmethane addition; Group B receiving only the antibiotic | In group A there was a significant improvement in NIH-CPSI (−17.6 ± 2.65) and IPSS (−12.2 ± 2.33) scores versus Group B (mean difference: −9 ± 1.82; −8.33 ± 1.71, respectively) | [24] |
| Morgia et al. | 2010 | 102 patients suffering from IIIa CP/CPPS, aged 23–49 years | Patients were randomly assigned into two groups: group A receiving Profluss (S. repens, selenium, and lycopene) or group B taking S. repens alone for two months | The NIH-CPSI score significantly improved (p < 0.001) in both groups; the decrease in IPSS score and improvement in the maximum peak flow rate was seen in both arms, but was more pronounced in group A. The decrease of PSA and WBC count (p < 0.007) was only reported in group A | [23] |
| Morgia et al. | 2013 | 168 patients suffering from BPH submitted to prostate biopsy for PCa suspicion. Two additional cores were taken for PCI evaluation | The first group consisted of 108 participants with histological diagnosis of PCI randomized to Profluss group (I) or to control group (Ic). The second group consisted of 60 participants with histological diagnosis of BPH, randomized to Profluss + α-blocker treatment group (II) or to the control group (IIc) | Alleviation of inflammatory state, decrease in mean values of interleukins (CD20, CD3, CD68), and mean PSA levels in group I compared to group Ic. The extension and grading of inflammatory state in group II were also decreased compared to IIc, but not statistically significantly. A statistically significant difference in interleukin levels (CD20, CD3, CD68, CD8) was reported in group II compared to IIc | [22] |
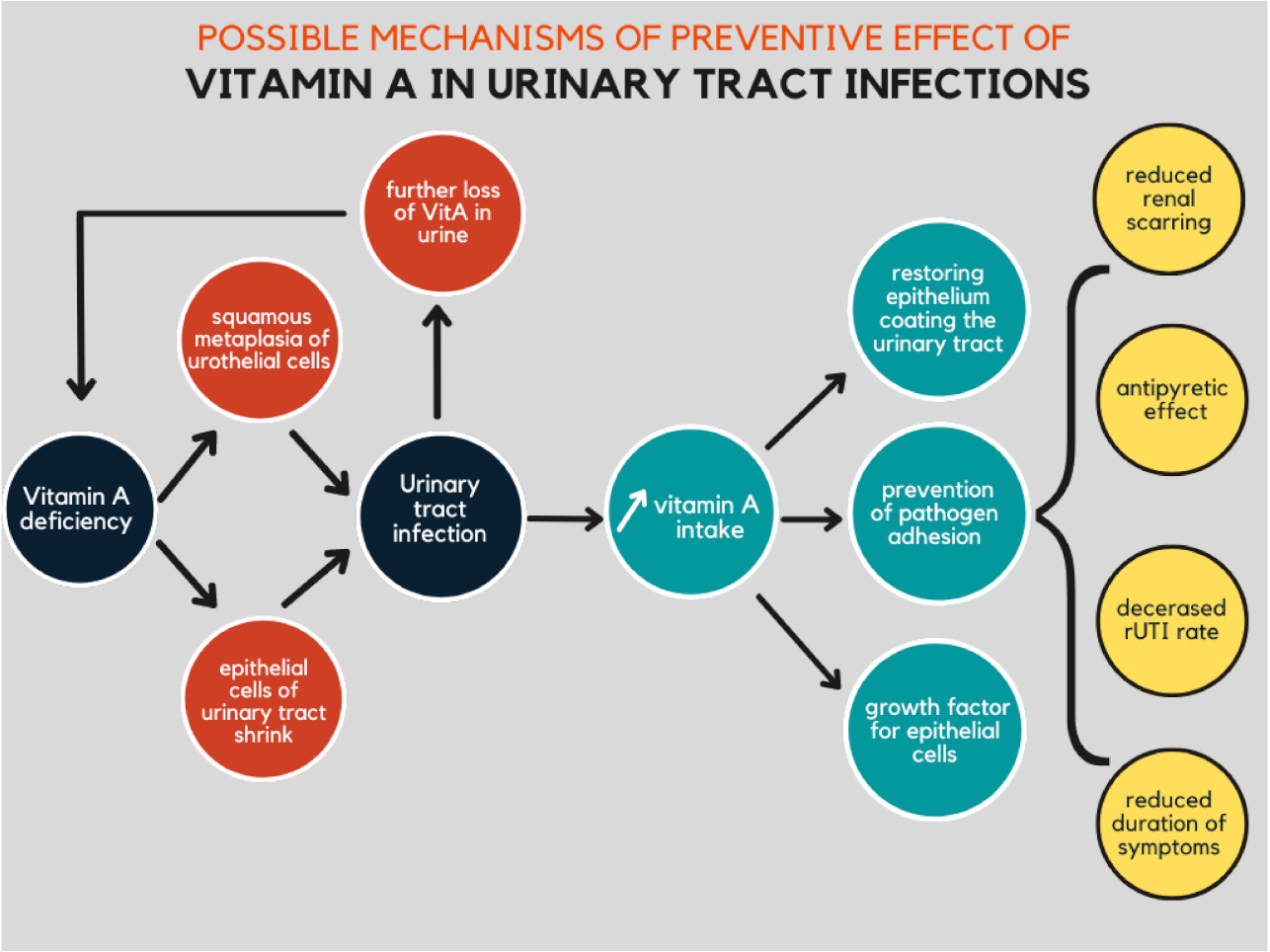
3. Urinary Tract Infection
3.1. Urinary Tract Infection Experimental Studies
3.2. Urinary Tract Infection Clinical Studies
| Study | Year | Material | Intervention | Results | Ref. |
|---|---|---|---|---|---|
| Kahbazi et al. | 2017 | 90 females aged 2–12 years diagnosed with UTIs and the first episode of APN | Participants were randomized into two groups: in addition to antibiotics the intervention group was given 10 days of oral vitamin A while the control group received 10 days of placebo | Duration of symptoms (fever, urinary frequency, and poor feeding) was significantly reduced in the intervention group. The second 99mTc-DMSA scan revealed worsening of patients’ kidney status in 22.2% of participants in the vitamin A group and 44.7% of patients in the placebo group (p = 0.003) | [35] |
| Yilmaz et al. | 2007 | 24 patients with uncomplicated rUTI were included | Patients were randomized into two groups: the first receiving a single dose of 200,000 IU vitamin A in addition to antibiotic treatment and the second being a control group | In the six months after treatment, the chance of suffering rUTI reduced from 3.58 to 0.75 in the intervention group. UTIs were statistically less frequent during the six months follow-up after vitamin A supplementation compared to the control group | [37] |
This entry is adapted from the peer-reviewed paper 10.3390/nu14040859
References
- Steers, W.D. 5α-Reductase Activity in the Prostate. Urology 2001, 58, 17–24.
- Lepor, H. Medical Treatment of Benign Prostatic Hyperplasia. Rev. Urol. Dis. State Rev. 2011, 13, 20–33.
- Hirshburg, J.M.; Kelsey, P.A.; Therrien, C.A.; Gavino, A.C.; Reichenberg, J.S. Adverse Effects and Safety of 5-Alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review. J. Clin. Aesthetic Dermatol. 2016, 9, 56.
- Dunn, C.J.; Matheson, A.; Faulds, D.M. Tamsulosin. Drugs Aging 2002, 19, 135–161.
- Clinton, S.K.; Emenhiser, C.; Schwartz, S.J.; Bostwick, D.G.; Williams, A.W.; Moore, B.J.; Erdman, J.W.; El, J.W. Cis-Trans Lycopene Isomers, Carotenoids, and Retinol in the Human Prostate. Cancer Epidemiol. Biomark. Prev. 1996, 5, 823.
- Palan, P.; Naz, R. Changes in Various Antioxidant Levels in Human Seminal Plasma Related to Immunoinfertility. Arch. Androl. 1996, 36, 139–143.
- Siler, U.; Barella, L.; Spitzer, V.; Schnorr, J.; Lein, M.; Goralczyk, R.; Wertz, K. Lycopene and Vitamin E Interfere with Autocrine/Paracrine Loops in the Dunning Prostate Cancer Model. FASEB J. 2004, 18, 1019–1021.
- Tavani, A.; Longoni, E.; Bosetti, C.; Maso, L.D.; Polesel, J.; Montella, M.; Ramazzotti, V.; Negri, E.; Franceschi, S.; Vecchia, C. la Intake of Selected Micronutrients and the Risk of Surgically Treated Benign Prostatic Hyperplasia: A Case-Control Study from Italy. Eur. Urol. 2006, 50, 549–554.
- Kristal, A.R.; Arnold, K.B.; Schenk, J.M.; Neuhouser, M.L.; Goodman, P.; Penson, D.F.; Thompson, I.M. Dietary Patterns, Supplement Use, and the Risk of Symptomatic Benign Prostatic Hyperplasia: Results from the Prostate Cancer Prevention Trial. Am. J. Epidemiol. 2008, 167, 925–934.
- Obermüller-Jevic, U.C.; Olano-Martin, E.; Corbacho, A.M.; Eiserich, J.P.; van der Vliet, A.; Valacchi, G.; Cross, C.E.; Packer, L. Lycopene Inhibits the Growth of Normal Human Prostate Epithelial Cells In Vitro. J. Nutr. 2003, 133, 3356–3360.
- Bonvissuto, G.; Minutoli, L.; Morgia, G.; Bitto, A.; Polito, F.; Irrera, N.; Marini, H.; Squadrito, F.; Altavilla, D. Effect of Serenoa Repens, Lycopene, and Selenium on Proinflammatory Phenotype Activation: An In Vitro And In Vivo Comparison Study. Urology 2011, 77, 248.e9–248.e16.
- Herzog, A.; Siler, U.; Spitzer, V.; Seifert, N.; Denelavas, A.; Hunziker, P.B.; Hunziker, W.; Goralczyk, R.; Wertz, K. Lycopene Reduced Gene Expression of Steroid Targets and Inflammatory Markers in Normal Rat Prostate. FASEB J. 2005, 19, 1–24.
- Minutoli, L.; Altavilla, D.; Marini, H.; Rinaldi, M.; Irrera, N.; Pizzino, G.; Bitto, A.; Arena, S.; Cimino, S.; Squadrito, F.; et al. Inhibitors of Apoptosis Proteins in Experimental Benign Prostatic Hyperplasia: Effects of Serenoa Repens, Selenium and Lycopene. J. Biomed. Sci. 2014, 21, 19.
- Kim, H.-S.; Bowen, P.; Chen, L.; Duncan, C.; Ghosh, L.; Sharifi, R.; Christov, K. Effects of Tomato Sauce Consumption on Apoptotic Cell Death in Prostate Benign Hyperplasia and Carcinoma. Nutr. Cancer 2003, 47, 40–47.
- Wang, L.; Hou, Y.; Wang, R.; Pan, Q.; Li, D.; Yan, H.; Sun, Z. Inhibitory Effect of Astaxanthin on Testosterone-Induced Benign Prostatic Hyperplasia in Rats. Mar. Drugs 2021, 19, 652.
- Habermacher, G.M.; Chason, J.T.; Schaeffer, A.J. Prostatitis/Chronic Pelvic Pain Syndrome. Annu. Rev. Med. 2006, 57, 195–206.
- Pontari, M.A.; Ruggieri, M.R. Mechanisms in prostatitis/chronic pelvic pain syndrome. J. Urol. 2004, 172, 839–845.
- Zou, Y.; Sun, Q.; Li, J.; Yang, C.; Yang, J.; Zhang, L. Effects of E/Z Isomers of Lycopene on Experimental Prostatic Hyperplasia in Mice. Fitoterapia 2014, 99, 211–217.
- Shahed, A.R.; Shoskes, D.A. Oxidative Stress in Prostatic Fluid of Patients With Chronic Pelvic Pain Syndrome: Correlation With Gram Positive Bacterial Growth and Treatment Response. J. Androl. 2000, 21, 669–675.
- de Stefano, D.; Maiuri, M.C.; Simeon, V.; Grassia, G.; Soscia, A.; Cinelli, M.P.; Carnuccio, R. Lycopene, Quercetin and Tyrosol Prevent Macrophage Activation Induced by Gliadin and IFN-γ. Eur. J. Pharmacol. 2007, 566, 192–199.
- Han, C.H.; Yang, C.H.; Sohn, D.W.; Kim, S.W.; Kang, S.H.; Cho, Y.-H. Synergistic Effect between Lycopene and Ciprofloxacin on a Chronic Bacterial Prostatitis Rat Model. Int. J. Antimicrob. Agents 2008, 31, 102–107.
- Morgia, G.; Cimino, S.; Favilla, V.; Russo, G.I.; Squadrito, F.; Mucciardi, G.; Masieri, L.; Minutoli, L.; Grosso, G.; Castelli, T. Effects of Serenoa Repens, Selenium and Lycopene (Profluss®) on Chronic Inflammation Associated with Benign Prostatic Hyperplasia: Results of “FLOG” (Flogosis and Profluss in Prostatic and Genital Disease), a Multicentre Italian Study. Int. Braz. J. Urol. 2013, 39, 214–221.
- Morgia, G.; Mucciardi, G.; Galì, A.; Madonia, M.; Marchese, F.; di Benedetto, A.; Romano, G.; Bonvissuto, G.; Castelli, T.; Macchione, L.; et al. Treatment of chronic prostatitis/chronic pelvic pain syndrome category IIIA with Serenoa repens plus selenium and lycopene (profluss®) versus S. repens alone: An Italian randomized multicenter-controlled study. Urol. Int. 2010, 84, 400–406.
- Cai, T.; Tiscione, D.; Gallelli, L.; Verze, P.; Palmieri, A.; Mirone, V.; Bartoletti, R.; Malossini, G. Serenoa Repens Associated with Selenium and Lycopene Extract and Bromelain and Methylsulfonylmethane Extract Are Able to Improve the Efficacy of Levofloxacin in Chronic Bacterial Prostatitis Patients. Arch. Ital. Urol. E Androl. 2016, 88, 177.
- Epp, A.; Larochelle, A. Recurrent Urinary Tract Infection. J. Obstet. Gynaecol. Can. 2017, 39, e422–e431.
- GRIEBLING, T.L. Urologic diseases in america project: Trends in resource use for urinary tract infections in women. J. Urol. 2005, 173, 1281–1287.
- Scholes, D.; Hooton, T.M.; Roberts, P.L.; Stapleton, A.E.; Gupta, K.; Stamm, W.E. Risk Factors for Recurrent Urinary Tract Infection in Young Women. J. Infect. Dis. 2000, 182, 1177–1182.
- Kurutas, E.B.; Ciragil, P.; Gul, M.; Kilinc, M. The Effects of Oxidative Stress in Urinary Tract Infection. Mediat. Inflamm. 2005, 2005, 242–244.
- Gul, M.; Kurutas, E.; Ciragil, P.; Cetinkaya, A.; Kilinc, M.; Aral, M.; Buyukbese, M.A. Urinary Tract Infection Aggravates Oxidative Stress in Diabetic Patients. Tohoku J. Exp. Med. 2005, 206, 1–6.
- Jung, C.; Brubaker, L. The Etiology and Management of Recurrent Urinary Tract Infections in Postmenopausal Women. Climacteric 2019, 22, 242–249.
- Friedman, A.; Sklan, D. Antigen-Specific Immune Response Impairment in the Chick as Influenced by Dietary Vitamin A. J. Nutr. 1989, 119, 790–795.
- Chandra, R.K. Increased Bacterial Binding to Respiratory Epithelial Cells in Vitamin A Deficiency. BMJ 1988, 297, 834–835.
- McDowell, E.M.; DeSanti, A.M.; Newkirk, C.; Strum, J.M. Effects of Vitamin A-Deficiency and Inflammation on the Conducting Airway Epithelium of Syrian Golden Hamsters. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1990, 59, 231–242.
- Munday, J.; McKinnon, H.; Aberdein, D. Cystitis, Pyelonephritis, and Urolithiasis in Rats Accidentally Fed a Diet Deficient in Vitamin A. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 790–794.
- Kahbazi, M.; Sharafkhah, M.; Yousefichaijan, P.; Taherahmadi, H.; Rafiei, M.; Kaviani, P.; Abaszadeh, S.; Massoudifar, A.; Mohammadbeigi, A. Vitamin A Supplementation Is Effective for Improving the Clinical Symptoms of Urinary Tract Infections and Reducing Renal Scarring in Girls with Acute Pyelonephritis: A Randomized, Double-Blind Placebo-Controlled, Clinical Trial Study. Complementary Ther. Med. 2019, 42, 429–437.
- Dalirani, R.; Zoshk, Y.; Sharifian, M.; Mohkam, M.; Karimi, A.; Fahimzad, A.; Varzandefar, M. Kidney Diseases Role of Vitamin A in Preventing Renal Scarring After Acute Pyelonephritis. Iran J. Kidney Dis. 2011, 5, 320–323.
- Yilmaz, A.; Bahat, E.; Yilmaz, G.G.; Hasanoglu, A.; Akman, S.; Guven, A.G. Adjuvant Effect of Vitamin A on Recurrent Lower Urinary Tract Infections. Pediatrics Int. 2007, 49, 310–313.
