TENG-Based Self-Powered Neuroprosthetics is a neuroprosthetic system using a triboelectric nanogenerator as the power source to generator the current pulses required for neural stimulations. The thin-film triboelectric nanogenerator can be attached onto the heart or buried under the skin to convert the mechanical energy from the movement of organs, such as heart beat, or the hand tapping onto the skin to electrical current pulses. This system is promising to realize a fully self-powered neural modulation with much reduced device complexity.
- Triboelectric nanogenerator
- Self-Powered Neuroprosthetics
- thin-film TENG device
- high-frequency switch
- bennet doubler conditioning circuit
1. Introduction
Neuroprosthetics is a powerful toolkit, for clinical interventions of various diseases, affecting the central nervous or peripheral nervous systems by electrically stimulating different neuronal structures [1]. Deep brain stimulation (DBS), based on the electrical stimulation of deep structures within the brain, is clinically used for symptomatic treatment of motor-related disorders, such as Parkinson’s disease, dystonia, and tremor, and it is also under clinical development for other drug-resistant neurological disorders, such as depression, obsessive-compulsive disorder, and others [2]. Electrical stimulation of the central nervous system (CNS) and peripheral nervous system (PNS) can also be achieved by implanted neuroprosthetic devices in the spinal cord or peripheral nerves and muscles to restore sensory and motor function in a novel and promising field of therapeutic interventions termed “bioelectronics” [3,4,5,6,7]. To prolong the lifetime of the implanted devices, researchers have invested enormous enthusiasm and energy to develop the power sources for them [8,9,10,11]. One of the primary strategies is harvesting power from organs or the surrounding environment, which is also called a self-powered method. Currently, there are six major research directions for achieving this self-powered method by harvesting energy from our own body. Triboelectric nanogenerators (TENGs) and piezoelectric nanogenerators harvest energy from the motion of organs; optical devices, ultrasound devices, and electromagnetic coils receive energy wirelessly by a transcutaneous approach; and biofuel cells extract energy from the redox reaction of glucose and electrolytes in the gastrointestinal tract [12]. Among all these methods, a TENG is the onlymethod to achieve direct nerve stimulations [13,14,15,16,17,18,19,20,21], showing its capability in the realization of a self-powered neuroprosthetic system in the future, which is far beyond the application of energy harvesting and self-powered physical and chemical sensing [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55].
2. The Development of Triboelectric Nanogenerator (TENG)-Based Self-Powered Nerve Stimulation
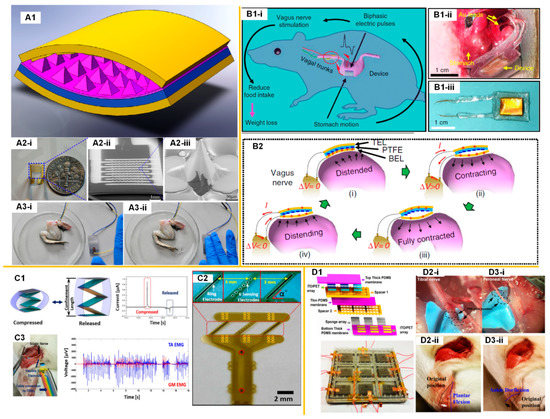
3. The Perspective for a TENG-Based Self-Powered Neuroprosthetic System
Although Yao et al. demonstrated a self-powered VNS system on a rat for a weight control application [14], this system could hardly meet the requirement for most neural modulation applications such as deep brain stimulation (DBS), vagus nerve stimulation (VNS), and functional electrical stimulation (FES) in humans. This is due to the fact that the current pulse frequency of TENG devices attached to the stomach and heart cannot be higher than 2 Hz, but the frequency of the current pulses required for a neuroprosthetic system is typically higher than 10 Hz. Therefore, the current demonstrated TENG-based neuroprosthetic system should be improved. Here, based on the state of the ongoing development of TENG-based nerve stimulation and relevant techniques, a perspective of the TENG-based self-powered neuroprosthetic system is proposed.
This TENG-based self-powered neuroprosthetic system consists of four major components, as shown in Figure 3. The first component is a thin film implantable TENG device, which requires a biocompatible package and can be driven by the heart, stomach, or hand tapping, if implanted beneath the skin. Since the frequency of the mechanical operation cannot fulfil the high-frequency current pulses required for neural modulation, a high-frequency switch is necessary to generate high-frequency current pulses (component 3 in Figure 3). The operation of this high-frequency switch can easily be achieved by a field-effect transistor (FET) or a relay, which is explained in detail in a subsequent section. However, when a single current pulse of low frequency is divided into a series of current pulses of high frequency, the amplitude of each current pulse decreases, and thus can be insufficient for nerve stimulations. Therefore, between components 1 and 3, an amplification circuit is required to amplify the output of the TENG device and ensure that each current pulse is high enough for nerve stimulations (component 2 in Figure 3). Then, the last component is the neural interface to enable the electrical nerve stimulation.
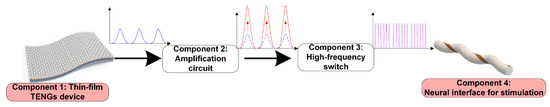
Figure 3. A perspective for the TENG-based self-powered neuroprosthetic system.
4. Summary
With proper device optimization, all kinds of nervous systems can be directly stimulated. The perspective of a further developed more sophisticated neuroprosthetic system is proposed, which includes a thin-film TENG device with a biocompatible package, an amplification circuit to enhance the output, and a self-powered high-frequency switch to generate high-frequency current pulses for nerve stimulations. The recent development and progress of each part are reviewed and evaluated. The scenario proposed in Figure 3 depicts the future of this TENG-based self-powered neuroprosthetic system, which is promising for the application of DBS, FES, and VNS.
This entry is adapted from the peer-reviewed paper 10.3390/mi11090865
