Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
|
Physics, Applied
The laser application for hyperthermia makes it possible to obtain managed thermal damage of tumor tissue. However, the small spatial selectivity of tumor tissue heating remains a problem of laser hyperthermia. The development of innovative nanoparticle-based technologies to improve the selectivity of laser heating is intensively pursued, and various types of plasmon resonance nanoparticles are used for this purpose, as follows: nanospheres nanoshells, nanorods, nanocages. Plasmonic photothermal therapy is referred to by the acronym PPT.
- oncology
- plasmonic photothermal therapy
- photodynamic therapy
- nanoparticles
1. The Nanoparticle Application for Antitumor Therapy
The application of nanoparticles as thermosensitizers for various types of hyperthermia has become a new trend in antitumor therapy. In oncology, electromagnetic radiation of different wavelengths, from radiofrequency to microwaves and ultrasonic waves, is used as heat sources for hyperthermia [1][2][3]. Hyperthermia is usually defined as the heating of a tissue to a temperature of 41–47 °C, less commonly temperatures in the range of 50–60 °C, for several minutes, which causes irreversible cellular damage through the appearance of disruptions in cell membranes and denaturation of proteins [4]. Tumor cells are more susceptible to hyperthermic effects than healthy cells because of their higher metabolic rate [5].
The laser application for hyperthermia makes it possible to obtain managed thermal damage of tumor tissue. However, the small spatial selectivity of tumor tissue heating remains a problem of laser hyperthermia [6][7]. The development of innovative nanoparticle-based technologies to improve the selectivity of laser heating is intensively pursued, and various types of plasmon resonance nanoparticles are used for this purpose, as follows: nanospheres [8] nanoshells [9], nanorods [10][11], nanocages [12][13]. In the current literature, plasmonic photothermal therapy is referred to by the acronym PPT [14].
The controlled synthesis of nanostructures allows adjusting the plasmon resonance of nanoparticles in a specific wavelength spectrum [15][16]. If the nanoparticles accumulate in the tumor tissue, the laser irradiation with a particular wavelength close to the plasmon resonance of gold nanoparticles causes thermolysis of tumor cells, while heating and damaging the surrounding healthy tissues does not occur [17][18]. For deeper tissue penetration of laser radiation, the tuning of the plasmon resonance of gold nanoparticles must be performed at the following wavelengths: 750–1100 nm, the so-called “therapeutic window of transparency” of tissues [19].
The passive and active delivery techniques are used to maximize the nanoparticle accumulation in the tumor tissue. Passive delivery is caused by the nanoparticle accumulation in the tumor tissue due to the effect of enhanced permeability and retention (“EPR effect”) characteristic of tumor vessels [20][21]. Disordered architectonics, arteriovenous shunts, changes in the shape and structure of endothelial cells, and enlarged pores in tumor vessels all make tumor vessels “perforated” [22][23]. Lack of functional lymphatic vessels in the tumor also reduces the elimination rate of nanoparticles from the tumor.
The effective and safe use of anticancer plasmonic photothermal therapy requires solving a number of problems related to the choice of the optimal method of nanoparticle delivery, reducing the nanoparticle accumulation in healthy tissues, developing methods to visualize their accumulation in the tumor, and optimizing therapy protocols [24][25][26]. The increase in nanoparticle accumulation in the tumor may be achieved by changing the size, shape, and coating of nanoparticles [27][28].
Thus, it was found that the use of polyethylene glycol (PEG) for nanoparticle coating reduces their agglomeration and increases their circulation time in the bloodstream [29]. For active delivery of nanoparticles, modification of their surface with tumor-specific markers was offered that can selectively bind to the membranes of tumor cells [30][31]. In research by O′Neal et al. [32], gold nanoshells were used for plasmonic photothermal therapy in mice with transplanted intestinal carcinoma; 6 h after intravenous injection of nanoparticles, 808 nm infrared laser with 4 W/cm2 power density was used to irradiate the tumor for 3 min, the authors noted a marked effect of therapy. Gobin et al. [33] intravenously injected gold nanoshells with an absorption maximum at 750–850 nm to mice with transplanted intestinal carcinoma, as follows: 20 h after injection, 808 nm infrared laser with 4 W/cm2 power density to irradiate the tumor for 3 min. On day 12 after exposure, a two-fold decrease in tumor volume and increased life expectancy of mice after photothermal therapy were detected.
Dickerson et al. [10] made the comparison of intratumoral and intravenous injection of gold nanorods in mice with squamous cell skin carcinoma. The authors noted that the most significant tumor accumulation of nanoparticles was observed 24 h after intravenous injection of gold nanorods, then this time point was applied for following PPT treatments for intravenous injections of AuNRs. It was found that 808 nm laser irradiation for 10–15 min at 1.7–1.9 W/cm2 was required for marked tumor destruction and minimal damage to surrounding tissues. However, a more pronounced effect was observed after intratumoral injection of gold nanorods on day 13 after photothermal treatment: the tumor volume decreased by 57% compared to the original volume, and with their intravenous injection it decreased by 23%.
Von Maltzahn et al. [34] found that at the same concentrations and PEG-coating gold nanorods are heated by laser irradiation six times faster than gold nanoshells. The same authors determined that PEG-coated gold nanorods circulate in the blood longer than PEG-coated nanoshells after intravenous injection. By intravenous infusion of polyethylene glycol-coated GNRs at a dose of 20 mg/kg in mice with melanoma xenograft MDA-MB-435, complete resorption of the tumor after photothermal therapy was achieved. This effect was probably due to the small tumor size and high dose of gold nanorods.
In work by Chen et al. [35], a high dose of gold nanocages (9 × 1012 particles) was applied for intravenous injection in mice with transplanted glioma line U78 to increase their tumor accumulation. Photothermal therapy using an infrared laser with a low power density (about 0.7 W/cm2) resulted in a significant decrease in the metabolic activity of tumor tissues. The treatment was performed under the control of positron emission tomography. Tae and coworkers [36] have demonstrated the pronounced antitumor effect of PTT in nude mice bearing bilateral SCC7 tumors using intravenous GNRs loaded Pluronic F 68 nanocarriers. The significant suppression of the tumor growth was observed after irradiating the tumors with 808 nm laser for 4 min, 24 h after injection of GNRs. El-Sayed et al. [37] studied the multiple intravenous administration of polyethylene glycol-coated gold nanorods, with an axial ratio of 4.6 and an absorption maximum of 800 nm (1.5 mg/kg once every three weeks) in Balb C mice with Ehrlich carcinoma. Three days after nanoparticle injection, the most significant accumulation of gold in the tumors was detected. One week after each intravenous injection, the tumor was irradiated for 2 min with a diode laser with a power density of 50 W/cm2, and the tumor heating was up to 79 °C. However, inhibition of tumor growth was noted by the authors only from days 22 to 47 of observation (Figure 1).
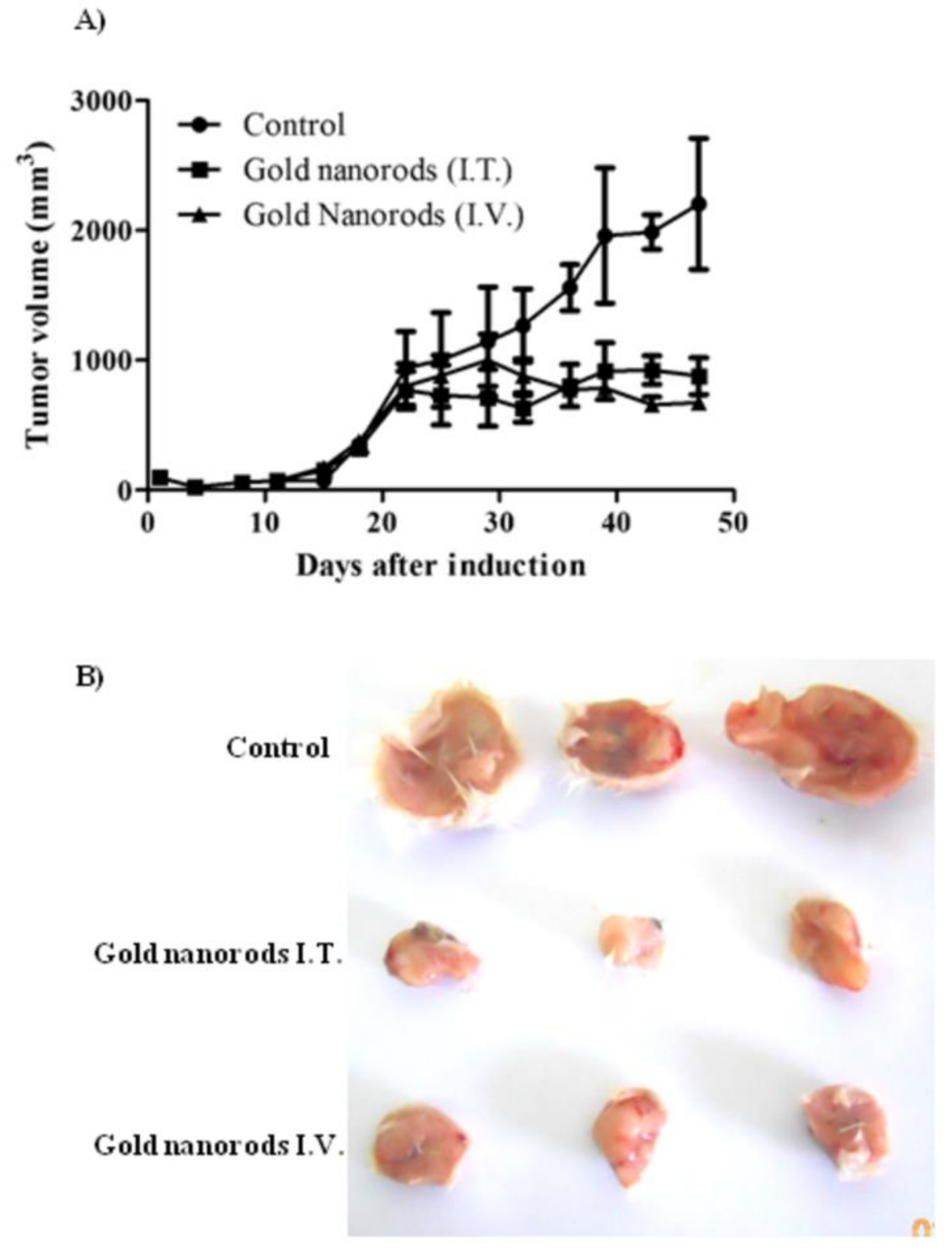
Figure 1. Antitumor activity of AuNRs coupled with laser-induced photo plasmonic thermal therapy in EACC solid tumor-bearing mice. EACC tumor-bearing mice were given gold NRs (1.5 mg/kg every three weeks) by I.V. (▲) and I.T. (■) administration compared to PBS-treated animals (●). Animals were exposed to a laser plasmonic beam (50 W/cm2 for 2 min) every week. Tumor size was measured every three days and plotted (A). Representative tumors are shown in panel (B).
In research by Sirotkina [38], gold nanorods of different sizes at a dose of 250 µg/kg (50/10 nm and 850 nm absorption maximum and 60/15 nm and 775 nm absorption maximum) were injected intravenously in CBA mice with cervical carcinoma. The nanoparticle accumulation in the tumor was visualized using OCT in dynamics for 7 h. At the highest accumulation of nanoparticles, the transplanted tumors were irradiated using a diode laser with a power density of 1.2–1.5 W/cm2, varying the laser power output to maintain the surface temperature of the transplanted tumor at 44–45 °C for 20 min. It is the case that 51% inhibition of the growth of tumors was observed one week after injection of 50/10 nm nanorods, after injection of 60/15 nm nanorods-the tumor growth inhibition rate was 72% [38]. Unfortunately, the small penetrating ability of OCT (2–3 mm) limits the use of OCT for visualization of nanoparticles accumulation in soft tissue tumors.
The greatest accumulation of gold in tumor tissue in rats with transplanted cholangiocarcinoma was detected after triple repeated intravenous injections of gold nanorods. The subsequent tumor irradiation by 808 nm laser with 2.3 W/cm2 power density for 15 min was conducted 24 h after the last IV injection of AuNRs. The significant damaging effect of PPT after triple IV injection of gold nanorods showed up in expressed necrotic and degenerative changes in tumors (Figure 2).
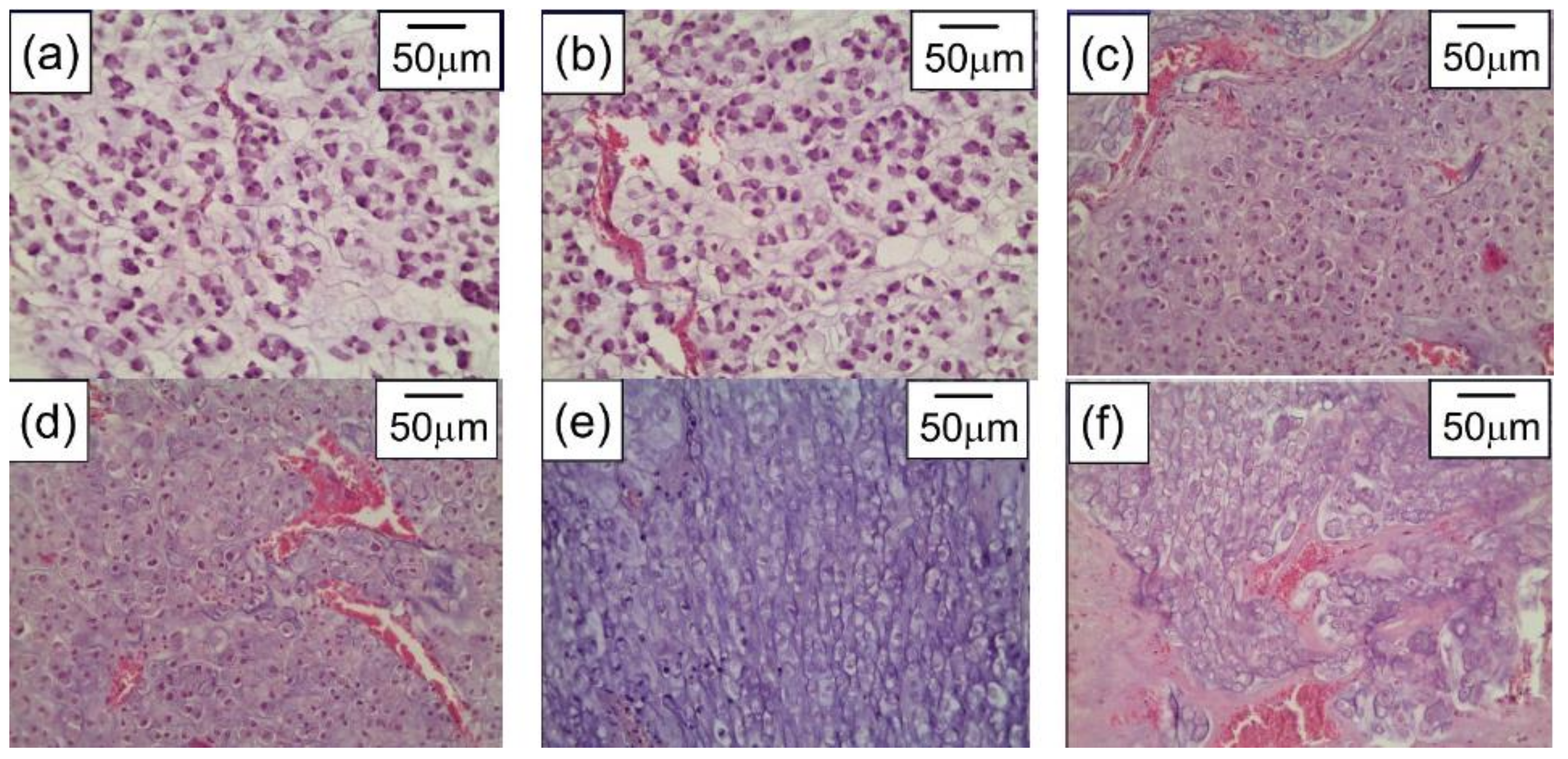
Figure 2. Cholangiocarcinoma PC-1–without any treatment (a), after only laser irradiation (b), after single IV administration of AuNRs and PPT (c), after double IV administration of AuNRs and PPT (d), after triple IV administration of AuNRs and PPT (e), and after intratumoral administration of AuNRs and PPT (f). H&E staining, magnification ×246.6.
However, if the accumulation of gold nanoparticles in the tumor tissue is insufficient, a slight temperature rise during laser hyperthermia can cause continued tumor growth. In addition, a significant problem is the optimal choice of time intervals between the intravenous injection of nanoparticles and the start of laser irradiation of the tumor to increase the efficiency of PPT therapy.
Doppler ultrasonography was conducted to describe transplanted tumors′ vascularity for optimal choice at the beginning of plasmonic photothermal therapy. AuNRs functionalized with thiolated polyethylene glycol (PEG) with an aspect ratio of 4.1 were used for multiple fractional intravenous administration in rats with transplanted cholangiocarcinoma PC-1. After the last injection of AuNRs, 808 nm laser irradiation of tumors, with 2.3 W/cm2 power density, was performed for 15 min by NIR diode laser LS-2-N-808-10000. The significant damage of tumor tissue with retardation of the tumor growth was observed after PPT treatment. The preliminary Doppler ultrasound allows to evaluate tumor vascularization and predict the efficiency of PPT depending on the degree of tumor vascularization (Figure 3).
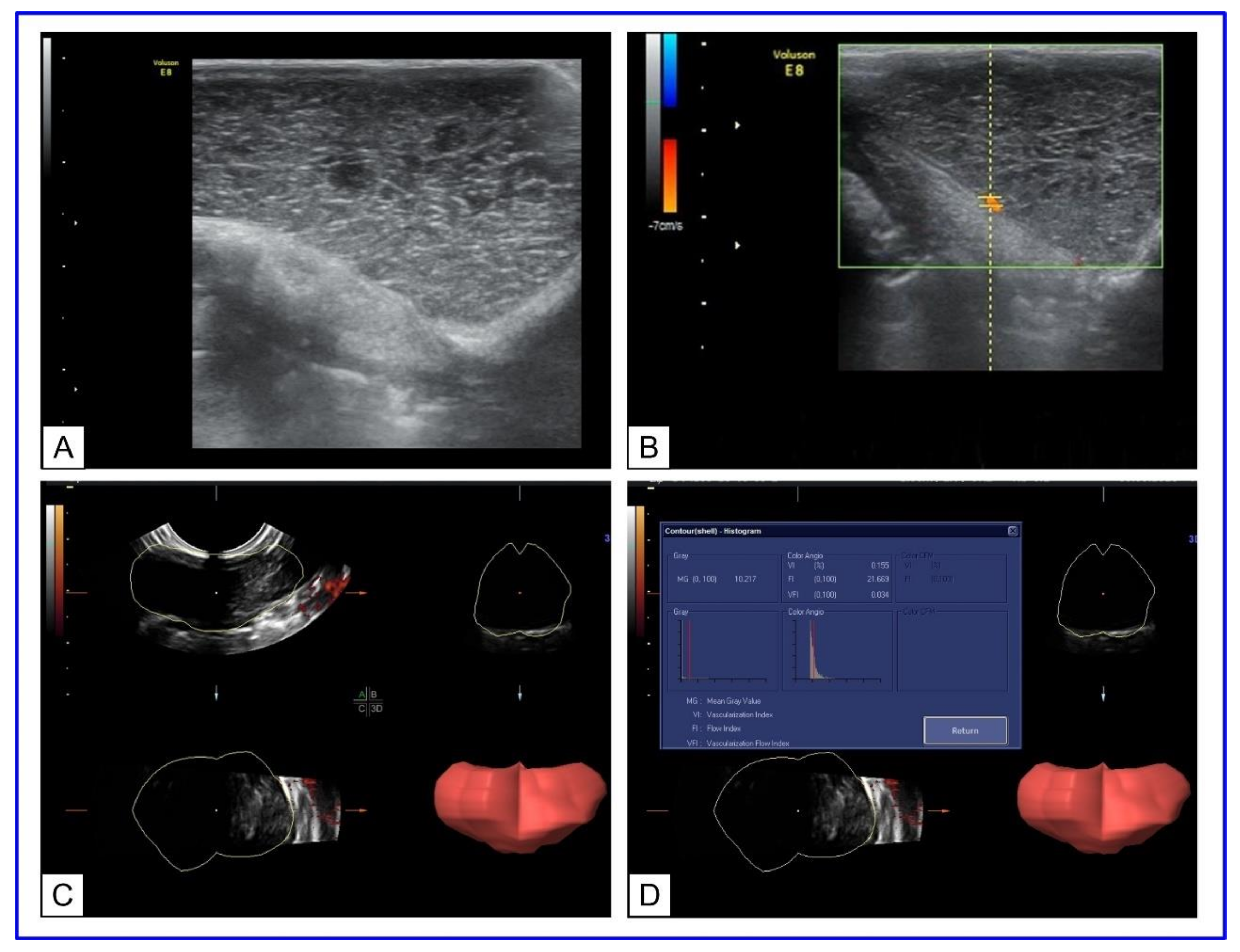
Figure 3. US Doppler imaging of rat tumors. (A) B-mode scanning; (B) 3D ultrasound mode. The lumen of the vessel is uniformly filled with color. (C) Three-dimensional (3D) power Doppler sonography in tumor-bearing rats. (D) Histogram analysis of vascularization indices.
A promising method of anticancer treatment is photodynamic therapy based on the use of photosensitizers [41]. After exposure to laser radiation with a certain wavelength, the photosensitizer goes to an excited state, and the formation of free radicals occurs, causing necrosis of tumor cells [42]. However, the major challenges of photodynamic therapy remain the achievement of the necessary accumulation of the photosensitizer in the tumor, the low penetrability of red laser radiation, insufficient oxygenation of many tumors, and slow biodegradation of photosensitizers [43][44]. The most commonly used photosensitizers are based on porphyrin [45][46], including Photosan® in Germany, Photofrin® in the USA and Canada, Alasens® and Photosens® in Russia. The use of metallic nanoparticles for conjugation with photosensitizers can develop combined methods of tumor therapy. The transition to the combined treatment of tumors using several alternative methods is a modern trend in experimental and clinical oncology [47][48]. A promising area of research is using a combination of phototherapy methods, namely, photothermal and photodynamic treatment for antitumor therapy. The use of laser radiation in both methods, acceleration of photochemical reactions during hyperthermia, synergistic effects on tumor blood vessels, time-difference of the therapeutic effects of PPT and PDT, increased thermosensitivity of cells in hypoxic conditions caused by the photochemical reaction-determine the effectiveness of combined therapy method [49][50][51]. Compared with conventional chemotherapy, combined phototherapy has significant advantages such as low invasiveness, high spatiotemporal selectivity, and rapid post-operation recovery [52][53].
Therefore, many research groups have proposed new treatment models that are based on the combination of PDT and PTT, using various types of nanoparticles and photosensitizers.
In 2014, Chen′s group proposed photosensitizer (Ce6)-loaded micelles integrated with cyanine dye for combined PTT/PDT treatment in mice with transplanted 4T1 tumors [54]. 24 h after intravenous injection of nanocomposites, the tumors suffered from various laser irradiation treatments, including PPT (785 nm, 5 min, 1.0 W/cm2), PDT (660 nm, 10 min, 1.0 W/cm2), combined PPT and PDT treatment (PTT/PDT), and reverse sequence of initial PDT and subsequent PTT treatment (PDT/PTT). The most pronounced tumor inhibition effect (~90%) was observed under sequential PTT/PDT treatments.
Zheng′s group proposed internalized RGD-modified indocyanine green (ICG) liposomes for PTT/PDT treatment of mice breast tumors 4T1. After 24 h intravenous injection of liposomes, the tumors of mice were irradiated by the 808 nm laser at a power density of 1.0 W/cm2 for 10 min. The tumor growth was significantly inhibited (up to ~98%), achieving almost complete tumor regression [55]. In 2018, Shen′s group reported that the assembly of iron oxide carbon dot NPs conjugated with black phosphorus quantum dots exhibited significant tumor-inhibition efficacy due to the synergistic PTT and PDT treatment via a near-infrared laser [56].
In combined photothermal and photodynamic therapy, several studies use nanocomposites with a gold core, which determines the heating during PPT, and a silicon shell containing encapsulated photosensitizer molecules responsible for the photochemical reaction. The common photosensitizers used in conjunction with the plasmonic NPs include phthalocyanines [57], toluidine blue, indocyanine green, and porphyrin derivatives [58]. Low toxicity of such nanocomposites was noted in a number of in vitro studies [59][60][61], a large number of articles are devoted to their in vivo application [62][63][64][65]. Jang B. et al. [62] developed gold nanorods conjugated with aluminum phthalocyanine tetrasulfonate (AuNR−AlPcS4). After intravenous injection of AuNR−AlPcS4 complex, tumor growth reduced by 79% with photodynamic therapy (PDT) alone and by 95% with dual photothermal therapy (PTT) and PDT in tumor-bearing mice. Wang S. et al. [63] reported a combined PDT/PTT treatment, in which MDA-MB-435 tumor-bearing mice received an intratumoral injection of gold nanostars conjugated with chlorin e6 (GNS-PEG-Ce6), followed by 6 min of 671 nm laser irradiation at 1.0 W/cm2 at four h post-injection. The early phase PDT effect was coordinated with the late-phase photothermal effect, indicating the high heat conversion and remarkable anticancer efficiency of Ce6-conjugated gold nanostars.
Nanocomposites (NCs), consisting of a gold nanorod core and a mesoporous silica shell doped with hematoporphyrin, have been fabricated for combining photothermal and photodynamic therapy (PDT + PTT) in rats with transplanted cholangiocarcinoma. NCs were directly injected into tumors and irradiated simultaneously with 633 nm and 808 nm lasers. In the comparison group with only PDT, weak changes in tissue histology and a moderate 20% decrease in the tumor volume were observed. In contrast, the combined PDT + PTT treatment resulted in large-area tumor necrosis (Figure 4) and dramatically decreased tumor volume. Zang S. et al. [65] proposed silica-coated gold nanorods conjugated with 4-carboxyphenyl porphyrin (AuNR@SiO2-TCPP) for combined photothermal and photodynamic therapy in mice with A549 xenograft tumors. The tumor growth in mice receiving AuNR@SiO2-TCPP with subsequent 660 and 808 nm irradiations was significantly inhibited, and the average tumor volume was decreased almost ten-fold compared to the control group.
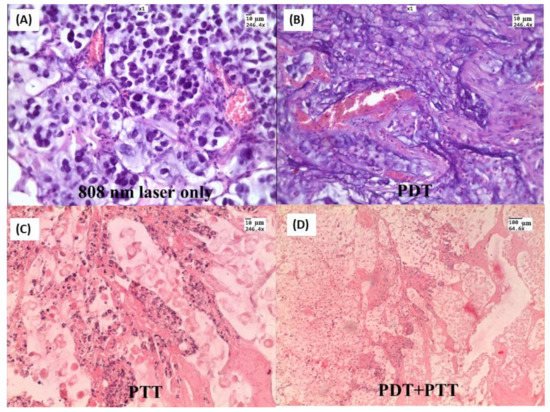
Figure 4. H&E-stained tumor slices after different treatments: (A) Laser 808 nm 2.3 W/cm2 treated only; (B) NCs injected and 633 nm 160 mW/cm2 treated; (C) NCs injected and 808 nm 2.3 W/cm2 treated; (D) NCs injected and simultaneously irradiated with two lasers. Tumor tissue specimens were obtained three days after treatment.
Compared with other functional nanomaterials for combined PDT/PPT therapy, the gold NPs demonstrated comparable biocompatibility and tumor inhibition performance, implying a promising potential for cancer therapeutics. However, the common combination of PDT and PTT needs to be activated by two separate lasers with different excitation wavelengths, which results in the prolongation of treatment time and the complication of the therapeutic process. Therefore, it is necessary to develop nanocomposites irradiated by a single wavelength laser to generate hyperthermia and ROS, triggering PPT and PDT simultaneously.
In recent work, Liu et al. [66] designed nanocomposites based on gold nanorods surface-functionalized with chlorin e6-C-15-ethyl ester (HB) and tumor-targeting peptide cyclic RGD (cRGD) to develop HB-AuNRs@cRGD for single NIR laser-induced targeted PDT/PTT. After intravenous administration in BALB/c female nude mice with ECA109 esophageal cancer model, the HB-AuNRs@cRGD could be preferentially accumulated within tumor sites and rapidly internalized by cancer cells. The pronounced damage of tumor cells with nuclear membrane fragmentation (karyorrhexis) and nuclei shrinkage with pyknosis was observed from tumor tissues in the HB-AuNRs@cRGD group with 660 nm laser irradiation, which could induce intensive necrosis or apoptosis, the tumor inhibition rate was approximately 77.04% [67].
Although phototherapy based on a combination of PDT/PPT treatments has been widely studied, many projects are still in the early preclinical stage, and there are still many deficiencies that need to be improved. Several unresolved problems are associated with the use of nanostructures for combined therapy, including those related to the choice of optimal doses and methods of nanocomposite administration, laser radiation protocols, and the most effective combinations of therapeutic effects. In addition, the safety, biocompatibility, and efficient targeting of tumor cells by PTT and PDT based on nanoparticles must be considered, and the clinical applicability for the treatment of various diseases must be further investigated.
2. Evaluation of Optical Properties of Tumors and the Propagation of Laser Radiation and Heat in Models Sensitized with AuNPs
Modeling the conversion of energy from light to heat by nanoparticles and the heating of the tissue surrounding the particles, as well as its kinetics, is important for the development of PPT. The efficiency of such energy conversion of gold and some other metallic nanoparticles under optical irradiation was evaluated using Monte Carlo modeling, described in Refs. [67][68]. The modeling of temperature fields in tumors with embedded AuNPs during PPT and model experiments with intratumorally injected nanoparticles have shown that the heating of nanoparticles and surrounding tissues is a fast enough process. For example, at the laser pulse duration of 2 ns and the intensity of 5 mW/cm2 at the plasmon resonant wavelength, the temperature increment in the gold nanoparticle reaches about 100 °C [69]. In Ref. [70], rapid heating of nanoparticles (picoseconds), which caused the initial peak of the photothermal signal, was detected. The increase in laser intensity led to the formation of nanobubbles around the superheated nanoparticles. The method of selective cancer cell thermomechanical destruction during laser irradiation at the wavelength of plasmonic resonance was patented [71].
Traditionally, the transport of radiation in tissue can be modeled either on the basis of a diffusion approximation approach or on the basis of stochastic analysis that takes into account the statistical uncertainty of radiation propagation in tissue. The analytical approach in combination with the Arrhenius damage integral was used by Yakunin et al. [72] for accurate quantitative assessment of hyperthermia and biological effects in conditions when the exposure time varied from tens to hundreds of seconds, and the generated heat affected an area of tens to hundreds of nanometers near the nanoparticle. In Ref. [73], nanoparticles were embedded subcutaneously at a depth of 1 mm, and the maximum temperature at the spot center after continuous laser irradiation with the intensity of 10 W/cm2 during 30 s achieved 65 °C. At this temperature, for perfusion rates typical of the cutaneous covering, the perfusion affected the thermal regime only at times longer than 60 s [74].
In most models, uniform distribution of the nanoparticles in tumors after the intratumoral injection is assumed. Therefore, the tumor is modeled as one object with homogeneous optical properties. However, knowledge of the real distribution of nanoparticles in tumors allows for correcting heat transport modeling during PPT. Von Maltzahn et al. [34] used noninvasive X-ray computed tomography or ex vivo spectrometry to obtain AuNR biodistribution data in tumors after intratumoral or intravenous administration, respectively, and four-dimensional computational heat transport modeling to predict real photothermal heating.
The use of PPT in antimicrobial therapy is widely studied [75][76][77][78][79]. Results of the photothermal effect of AuNPs on microorganisms have shown to be dependent on the shape and optical properties of the nanoparticles and properties of laser radiation [80]. The formation of a local temperature field in suspensions of microorganisms with embedded AuNRs under irradiation with a NIR laser (power density of around 100 mW/cm2) was considered theoretically in Ref. [81]. It was found that AuNPs functionalized by human immunoglobulins IgA and IgG formed “clouds” around cells and induced temperature rise in the microscale zone.
Knowledge of the optical properties of tumors is beneficial for analyses and optimization of the parameters determining laser energy absorption. Many research teams are working in this direction. The optical properties of various tumors in a wide wavelength range have been reviewed in Ref. [82].
Typical parameters of laser irradiance at the surface during PPT are the following: 2–50 W/cm2 power density, 1–5 mm spot size, and a wide range of heating duration (from shorter than several minutes to longer than 15 min) [32][33][35][36][37][38][39][40][64][83]. These parameters are used for computational Monte Carlo simulation of laser irradiation in tumors. For example, a Monte Carlo algorithm was developed by Manuchehrabadi et al. [84] to simulate photon propagation (808 nm, 1.6 W/cm2) in a spherical tumor after intratumoral injection of AuNRs calculate the absorption of laser energy in the tumor and investigate the effect of absorption and scattering coefficients on the simulated heating.
The change of the penetration depth of the laser light (1064 nm) in a tissue model with three layers of skin, adipose tissue, and muscle, before and after application of glycerol, were investigated by Youn [85]. By substituting the absorption and scattering coefficient into a numerical simulation of the temperature distribution, it was found that the application of optical immersion agents during laser treatment can reduce heat generation on the skin surface and stimulate the tissue′s heating deeply. The simulation was confirmed by experimental data [85][86].
The thermal effect on tumor tissues at different depths can be assessed by measuring the optical parameters of irradiated and non-irradiated tissues. Until now, this problem remains insufficiently studied. The changes in the optical parameters of biological tissues under the influence of temperature have been presented in a lot of works, for example, for blood [87], brain [88][89], skin [90][91], prostate [92], liver [93][94], etc. However, changes in the optical parameters of tumors during PPT require detailed research.
It should be noted that the main accumulation of nanoparticles during intravenous injection occurs in the blood vessels of the tumor, which are localized mainly in the peripheral part of the tumor. The intratumoral injection also does not allow for a uniform distribution of particles inside the tumor. Thus, the heating of the tumor tissue occurs unevenly. To optimize the laser exposure, information about the heating of the inner layers of tumor tissue in vivo is required. Optical properties of main layers of model cholangiocarcinoma in rats (capsule, top part, center, and bottom part) and also bordering layers of skin and subcutaneous tissue were evaluated in the spectral range 350–2200 nm before and after PPT. In these studies, AuNRs were injected intravenously or intratumorally with different doses, and IR diode laser (808 nm, 2.3 W/cm2) was used for irradiation. In Ref. [95], combined use of an immersion agent with low-intensity laser irradiation (1.5 W/cm2) for optical clearing of the skin before PPT was proposed. The effect of immersion agent (mixture of 70%-glycerol and 10%-dimethyl sulfoxide) on the optical parameters of the skin, subcutaneous tissue, and a model tumor in rats in vivo after hyperthermia caused by PPT was presented as a pilot result.
3. Combining PPT with Other Therapies to Achieve Synergic Efficiency
One of the most promising approaches is the integration of PPT with other techniques to achieve advanced synergic anticancer therapy. There are at least five possible ways for such integration: (1) PTT + PDT; PTT + chemotherapy (PTT + CHT); (3) PTT + immunotherapy (PTT + IMT); (4) PTT + gene therapy (PTT + GT); (5) PTT + radiotherapy (PTT + RT) [96]. An interesting PPT/PDT agent is pCo3O4 nanoplates. Because of the unique properties of Co3O4 NPs, their PTT/PDT and other functions are in ongoing examinations in many laboratories worldwide. In particular, Yuan et al. [97] reported multifunctional abilities of pCo3O4 NPs as photoacoustic/magnetic resonance contrast-imaging agents and as NIR-triggered PTT/PDT therapeutic nanoformulations. Furthermore, they also confirmed the suppression of the epithelial–mesenchymal transition (EMT) pathway by pCo3O4 NPs.
In recent years, several groups reported the unusual ability of AuNRs [98][99][100], AuNCGs [101], AuNSHs [102], and bipyramids [103] to generate singlet oxygen, thus enabling PDT treatment. Because of plasmonic absorption, all the above NPs would be suitable for combined PPT/PDT therapy. Unfortunately, there are only a few reports on the subject. Therefore, it is not clear whether this approach can find experimental confirmation. In any case, a close examination of reported ABDA oxidation plots clearly points to a low efficiency in ROS producing under irradiation of bare Au nanorods or bipyramids. Au atomic nanoclusters can produce singlet oxygen under laser irradiation. However, the level of delivered singlet oxygen concentration is much lower than can be observed with common PDT photosensitizers. Therefore, including the common effective PDT agents such as chlorin e6 into therapeutic composites seems to be a more promising way. To summarize, in spite of attractive properties [104], the combined PPT/PDT approach can suffer from the limited NIR irradiation in deep tissue layers. In addition, the excitation wavelengths for PTT and PDT sensitizers can be different, thus reducing the efficiency of combined therapy. Therefore, further research is needed in this area.
According to the literature data [99][105] (still a few), the combined use of PTT and CHT can cause a number of positive effects: (1) accelerate the penetration of drugs into cancer cells; (2) reduce the side effects of CHT (3); remove cancer tissues more effectively; (4) reduce multidrug resistance. The most striking manifestation of the synergism of PTT and CHT is the direct enhancement of the cytotoxicity of well-known drugs used in oncology, such as cisplatin, carboplatin, cyclophosphamide, carmustine. When combined with PPT and an increase in temperature from 37 to 40 degrees, a linear increase in cytotoxicity was observed [96]. In a study by Urano et al. [106], a significant increase in cytotoxicity was observed with an increase in temperature in the range of 40.5–43 degrees. The ineffectiveness of PTT may be associated with the activation of heat shock proteins (HSPs), leading to an increase in the resistance of cancer cells to heat. Under conditions of a heterogeneous tumor and a strong attenuation of radiation in deep tissue layers, activation of HSPs can minimize the effect of PTT. On the other hand, the inclusion of several chemotherapy drugs in one nanoformulation can increase the synergistic effect of PTT and reduce the side effects of therapy.
Compared to radiation therapy and chemotherapy, IMT relies on the use of the patient′s immune system. Typically, the patient′s immune system is unable to respond with a protective response against tumor development because cancer cells reduce the stimulation of T lymphocytes [107]. When various immune cells, including dendritic cells and T cells, invade the lesions, the activation of this antigen-specific immune response is mainly suppressed due to the immunosuppressive effect of TME. The method of immunotherapy is based on the activation of immune cells capable of infiltrating a tumor by triggering an antigen-specific immune response, since cancer cells at the site of the tumor release specific antigens. Interestingly, PDT treatment of cancer can induce immunogenic call death [108]. Although there are several reports on the successful integration of PTT and IMT [96][109][110][111], the lack of clear understanding of all details of PPT-modulated immune response is an open question.
The use of GT in cancer therapy is based on triggering apoptosis of tumor cells, increasing the level of cytotoxic immune cytokines, and suppressing HSP expression. In addition, cellular uptake of DNA or RNA is usually tricky due to their degradation by enzymes. Delivery of DNA and RNA using photothermally inducible agents may solve this problem [112]. In addition, PTT can induce improved gene silencing due to enhanced endosomal escape of gene delivery vectors [113]. The main obstacles to in-clinic translation of PTT + GT technology are biocompatibility and toxicity concerns, together with a small volume of experimental data.
In contrast to optically induced PDT and PTT technologies, RT has no depth limitation to kill tumors. That is why computer tomography-guided RT is a powerful clinic technology [114]. However, tumor hypoxia is the main obstacle in RT applications at low linear-energy transfer, typical for many photon/electron beams, because the O2 concentration controls the DNA damage by reactive oxygen species. Nevertheless, recent reports [115][116] showed promising capabilities of integrated RT and PPT technologies.
This entry is adapted from the peer-reviewed paper 10.3390/ma15041606
References
- Zhu, L.; Altman, M.B.; Laszlo, A.; Straube, W.; Zoberi, I.; Hallahan, D.E.; Chen, H. Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med. Biol. 2019, 45, 1025–1043.
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating Technology for Malignant Tumors: A Review. Int. J. Hyperth. 2020, 37, 711–741.
- Yagawa, Y.; Tanigawa, K.; Kobayashi, Y.; Yamamoto, M. Cancer Immunity and Therapy Using Hyperthermia with Immunotherapy, Radiotherapy, Chemotherapy, and Surgery. J. Cancer Metastasis Treat. 2017, 3, 218.
- Horsman, M.R. Tissue Physiology and the Response to Heat. Int. J. Hyperth. 2006, 22, 197–203.
- Labavić, D.; Ladjimi, M.T.; Courtade, E.; Pfeuty, B.; Thommen, Q. Mammalian Cell Sensitivity to Hyperthermia in Various Cell Lines: A New Universal and Predictive Description. Int. J. Hyperth. 2020, 37, 506–516.
- Habash, R.W.Y.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal Therapy, Part III: Ablation Techniques. Crit. Rev. Biomed. Eng. 2007, 35, 37–121.
- Priester, M.I.; Curto, S.; van Rhoon, G.C.; Hagen, T.L.M.T. External Basic Hyperthermia Devices for Preclinical Studies in Small Animals. Cancers 2021, 13, 4628.
- ElBialy, N.; Abdelhamid, M.; Youssef, T. Low Power Argon Laser-Induced Thermal Therapy for Subcutaneous Ehrlich Carcinoma in Mice Using Spherical Gold Nanoparticles. J. Biomed. Nanotechnol. 2010, 6, 687–693.
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic. Bioeng. Transl. Med. 2016, 1, 10–29.
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.H.; El-Sayed, I.H.; Chu, H.H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold Nanorod Assisted Near-Infrared Plasmonic Photothermal Therapy (PPTT) of Squamous Cell Carcinoma in Mice. Cancer Lett. 2008, 269, 57–66.
- Pan, S.; Xing, H.; Fu, X.; Yu, H.; Yang, Z.; Yang, Y.; Sun, W. The Effect of Photothermal Therapy on Osteosarcoma with Polyacrylic Acid–Coated Gold Nanorods. Dose-Response 2018, 16, 1559325818789841.
- Zhang, A.-W.; Guo, W.-H.; Qi, Y.-F.; Wang, J.-Z.; Ma, X.-X.; Yu, D.-X. Synergistic Effects of Gold Nanocages in Hyperthermia and Radiotherapy Treatment. Nanoscale Res. Lett. 2016, 11, 1–14.
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167.
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic Photothermal Therapy (PPTT) Using Gold Nanoparticles. Lasers Med. Sci. 2008, 23, 217–228.
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iatì, M.A. Surface Plasmon Resonance in Gold Nanoparticles: A Review. J. Physics Condens. Matter 2017, 29, 203002.
- Terentyuk, G.S.; Maslyakova, G.; Suleymanova, L.V.; Khlebtsov, N.; Khlebtsov, B.; Akchurin, G.G.; Maksimova, I.L.; Tuchin, V. Laser-Induced Tissue Hyperthermia Mediated by Gold Nanoparticles: Toward Cancer Phototherapy. J. Biomed. Opt. 2009, 14, 021016.
- Choi, W.I.; Sahu, A.; Kim, Y.H.; Tae, G. Photothermal Cancer Therapy and Imaging Based on Gold Nanorods. Ann. Biomed. Eng. 2011, 40, 534–546.
- Harris, N.; Ford, A.M.J.; Cortie, M.B. Optimization of Plasmonic Heating by Gold Nanospheres and Nanoshells. J. Phys. Chem. B 2006, 110, 10701–10707.
- Hainfeld, J.F.; O′Connor, M.J.; Lin, P.; Qian, L.; Slatkin, D.N.; Smilowitz, H.M. Infrared-Transparent Gold Nanoparticles Converted by Tumors to Infrared Absorbers Cure Tumors in Mice by Photothermal Therapy. PLoS ONE 2014, 9, e88414.
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tu-moritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392.
- Maeda, H. Toward a Full Understanding of the EPR Effect in Primary and Metastatic Tumors as Well as Issues Related to Its Heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6.
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771.
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to Drug Delivery in Solid Tumors. Tissue Barriers 2014, 2, e29528.
- Khlebtsov, N.; Dykman, L. Biodistribution and Toxicity of Engineered Gold Nanoparticles: A Review of in Vitro and in Vivo Studies. Chem. Soc. Rev. 2011, 40, 1647–1671.
- Bucharskaya, A.B.; Maslyakova, G.N.; Afanasyeva, G.A.; Terentyuk, G.S.; Navolokin, N.A.; Zlobina, O.V.; Chumakov, D.S.; Bashkatov, A.N.; Genina, E.; Khlebtsov, N.G.; et al. The Morpho-Functional Assessment of Plasmonic Photothermal Therapy Effects on Transplanted Liver Tumor. J. Innov. Opt. Health Sci. 2015, 8, 1541004.
- Zhang, B.; Hu, Y.; Pang, Z. Modulating the Tumor Microenvironment to Enhance Tumor Nanomedicine Delivery. Front. Pharmacol. 2017, 8, 952.
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090.
- Durymanov, M.; Rosenkranz, A.; Sobolev, A.S. Current Approaches for Improving Intratumoral Accumulation and Distribution of Nanomedicines. Theranostics 2015, 5, 1007–1020.
- Glomm, W. Functionalized Gold Nanoparticles for Applications in Bionanotechnology. J. Dispers. Sci. Technol. 2005, 26, 389–414.
- Gholipourmalekabadi, M.; Mobaraki, M.; Ghaffari, M.; Zarebkohan, A.; Omrani, V.F.; Urbanska, A.M.; Seifalian, A. Targeted Drug Delivery Based on Gold Nanoparticle Derivatives. Curr. Pharm. Des. 2017, 23, 2918–2929.
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active Targeting of Gold Nanoparticles as Cancer Therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789.
- O′Neal, D.; Hirsch, L.R.; Halas, N.; Payne, J.; West, J.L. Photo-Thermal Tumor Ablation in Mice Using Near Infrared-Absorbing Nanoparticles. Cancer Lett. 2004, 209, 171–176.
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-Infrared Resonant Nanoshells for Combined Optical Imaging and Photothermal Cancer Therapy. Nano Lett. 2007, 7, 1929–1934.
- Von Maltzahn, G.; Park, J.-H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally Guided Photothermal Tumor Therapy Using Long-Circulating Gold Nanorod Antennas. Cancer Res. 2009, 69, 3892–3900.
- Chen, J.; Glaus, C.; Laforest, R.; Zhang, Q.; Yang, M.; Gidding, M.; Welch, M.J.; Xia, Y. Gold Nanocages as Photothermal Transducers for Cancer Treatment. Small 2010, 6, 811–817.
- Choi, W.I.; Kim, J.-Y.; Kang, C.; Byeon, C.C.; Kim, Y.H.; Tae, G. Tumor Regression In Vivo by Photothermal Therapy Based on Gold-Nanorod-Loaded, Functional Nanocarriers. ACS Nano 2011, 5, 1995–2003.
- El-Sayed, M.A.; Shabaka, A.A.; El-Shabrawy, O.A.; Yassin, N.A.; Mahmoud, S.S.; El-Shenawy, S.M.; Al-Ashqar, E.; Eisa, W.H.; Farag, N.M.; El-Shaer, M.A.; et al. Tissue Distribution and Efficacy of Gold Nanorods Coupled with Laser Induced Photoplasmonic Therapy in Ehrlich Carcinoma Solid Tumor Model. PLoS ONE 2013, 8, e76207.
- Sirotkina, M.A. Visualization and Laser Hyperthermia of Biological Tissues Using Gold Plasmon Resonance Nanoparticles. Ph.D. Thesis, Institute of Theoretical and Experimental Biophysics RAS, Pushchino, Russia, 2014.
- Bucharskaya, A.B.; Maslyakova, G.N.; Dikht, N.I.; Navolokin, N.A.; Terentyuk, G.S.; Bashkatov, A.N.; Genina, E.; Khlebtsov, B.; Khlebtsov, N.G.; Tuchin, V. Plasmonic Photothermal Therapy of Transplanted Tumors in Rats at Multiple Intravenous Injection of Gold Nanorods. BioNanoScience 2016, 7, 216–221.
- Bucharskaya, A.B.; Maslyakova, G.N.; Chekhonatskaya, M.L.; Terentyuk, G.S.; Navolokin, N.A.; Khlebtsov, B.N.; Khlebtsov, N.G.; Bashkatov, A.N.; Genina, E.A.; Tuchin, V.V. Plasmonic Photothermal Therapy: Approaches to Advanced Strategy. Lasers Surg. Med. 2018, 50, 1025–1033.
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.-S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, e1900132.
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626.
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 400.
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent Progress in Photosensitizers for Overcoming the Challenges of Photodynamic Therapy: From Molecular Design to Application. Chem. Soc. Rev. 2021, 50, 4185–4219.
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Kim, C.-H.; Seo, S.-Y.; Lee, K.H. Design and Synthesis of New Porphyrin Analogues as Potent Photosensitizers for Photodynamic Therapy: Spectroscopic Approach. J. Fluoresc. 2020, 30, 397–406.
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603.
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043.
- Błaszkiewicz, P.; Kotkowiak, M. Gold-Based Nanoparticles Systems in Phototherapy-Current Strategies. Curr. Med. Chem. 2019, 25, 5914–5929.
- Khlebtsov, B.; Panfilova, E.; Khanadeev, V.; Bibikova, O.; Terentyuk, G.; Ivanov, A.; Rumyantseva, V.; Shilov, I.; Ryabova, A.; Loshchenov, V.; et al. Nanocomposites Containing Silica-Coated Gold–Silver Nanocages and Yb–2,4-Dimethoxyhematoporphyrin: Multifunctional Capability of IR-Luminescence Detection, Photosensitization, and Photothermolysis. ACS Nano 2011, 5, 7077–7089.
- Chen, B.; Cao, J.; Zhang, K.; Zhang, Y.-N.; Lu, J.; Iqbal, M.Z.; Zhang, Q.; Kong, X. Synergistic Photodynamic and Photothermal Therapy of BODIPY-Conjugated Hyaluronic Acid Nanoparticles. J. Biomater. Sci. Polym. Ed. 2021, 32, 2028–2045.
- Pinto, A.; Pocard, M. Photodynamic Therapy and Photothermal Therapy for the Treatment of Peritoneal Metastasis: A Systematic Review. Pleura Peritoneum 2018, 3, 20180124.
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the Drawbacks of Photothermal and Photodynamic Cancer Therapy. Adv. Sci. 2021, 8, 2002504.
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674.
- Guo, M.; Mao, H.; Li, Y.; Zhu, A.; He, H.; Yang, H.; Wang, Y.; Tian, X.; Ge, C.; Peng, Q.; et al. Dual Imaging-Guided Photothermal/Photodynamic Therapy Using Micelles. Biomaterials 2014, 35, 4656–4666.
- Yan, F.; Wu, H.; Liu, H.; Deng, Z.; Liu, H.; Duan, W.; Liu, X.; Zheng, H. Molecular Imaging-Guided Photothermal/Photodynamic Therapy against Tumor by iRGD-Modified Indocyanine Green Nanoparticles. J. Control. Release 2015, 224, 217–228.
- Zhang, M.; Wang, W.; Cui, Y.; Zhou, N.; Shen, J. Near-Infrared Light-Mediated Photodynamic/Photothermal Therapy Nanoplatform by the Assembly of Fe3O4 Carbon Dots with Graphitic Black Phosphorus Quantum Dots. Int. J. Nanomed. 2018, 13, 2803–2819.
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as Medicinal Photosensitizers: Developments in the Last Five Years. Co-ord. Chem. Rev. 2019, 379, 147–160.
- Yang, Z.; Sun, Z.; Ren, Y.; Chen, X.; Zhang, W.; Zhu, X.; Mao, Z.; Shen, J.; Nie, S. Advances in Nanomaterials for Use in Photothermal and Photodynamic Therapeutics (Review). Mol. Med. Rep. 2019, 20, 5–15.
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat. Commun. 2019, 10, 1–16.
- Dykman, L.A.; Khlebtsov, N.G. Multifunctional Gold-Based Nanocomposites for Theranostics. Biomaterials 2016, 108, 13–34.
- Choi, J.; Lee, S.-E.; Park, J.-S.; Kim, S.Y. Gold Nanorod-Photosensitizer Conjugates with Glutathione-Sensitive Linkages for Synergistic Cancer Photodynamic/Photothermal Therapy. Biotechnol. Bioeng. 2018, 115, 1340–1354.
- Jang, B.; Park, J.-Y.; Tung, C.-H.; Kim, I.-H.; Choi, Y. Gold Nanorod−Photosensitizer Complex for Near-Infrared Fluorescence Imaging and Photodynamic/Photothermal Therapy In Vivo. ACS Nano 2011, 5, 1086–1094.
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv. Mater. 2013, 25, 3055–3061.
- Terentyuk, G.; Panfilova, E.; Khanadeev, V.; Chumakov, D.; Genina, E.; Bashkatov, A.; Tuchin, V.; Bucharskaya, A.; Maslyakova, G.; Khlebtsov, N.; et al. Gold Nanorods with a Hematoporphyrin-Loaded Silica Shell for Dual-Modality Photodynamic and Photothermal Treatment of Tumors in Vivo. Nano Res. 2014, 7, 325–337.
- Zhang, S.; Lv, H.; Zhao, J.; Cheng, M.; Sun, S. Synthesis of Porphyrin-Conjugated Silica-Coated Au Nanorods for Synergistic Photothermal Therapy and Photodynamic Therapy of Tumor. Nanotechnology 2019, 30, 265102.
- Liu, Z.; Xie, F.; Xie, J.; Chen, J.; Li, Y.; Lin, Q.; Luo, F.; Yan, J. New-Generation Photosensitizer-Anchored Gold Nanorods for a Single Near-Infrared Light-Triggered Targeted Photodynamic–Photothermal Therapy. Drug Deliv. 2021, 28, 1769–1784.
- Wang, L.; Jacques, S.L.; Zheng, L. MCML—Monte Carlo Modeling of Light Transport in Multi-Layered Tissues. Comput. Methods Programs Biomed. 1995, 47, 131–146.
- Wilson, B.C.; Adam, G. A Monte Carlo Model for the Absorption and Flux Distributions of Light in Tissue. Med. Phys. 1983, 10, 824–830.
- Avetisyan, A.Y.; Yakunin, A.N.; Tuchin, V.V. On the Problem of Local Tissue Hyperthermia Control: Multiscale Modelling of Pulsed Laser Radiation Action on a Medium with Embedded Nanoparticles. Quantum Electron. 2011, 40, 1081–1088.
- Shao, J.; Griffin, R.J.; Galanzha, E.I.; Kim, J.-W.; Koonce, N.; Webber, J.; Mustafa, T.; Biris, A.S.; Nedosekin, D.; Zharov, V.P. Photothermal Nanodrugs: Potential of TNF-Gold Nanospheres for Cancer Theranostics. Sci. Rep. 2013, 3, 1293.
- Oraevsky, A.; Lapotko, D. Laser Activated Nanothermolysis of Cells. U.S. Patent 13/136,939, 23 February 2012.
- Yakunin, A.N.; Avetisyan, Y.A.; Tuchin, V.V. Quantification of Laser Local Hyperthermia Induced by Gold Plasmonic Nanoparticles. J. Biomed. Opt. 2015, 20, 51030.
- Maksimova, I.L.; Akchurin, G.G.; Terentyuk, G.S.; Khlebtsov, B.; Ermolaev, I.A.; Skaptsov, A.A.; Revzina, E.M.; Tuchin, V.; Khlebtsov, N.G. Laser Photothermolysis of Biological Tissues by Using Plasmon-Resonance Particles. Quantum Electron. 2008, 38, 536–542.
- Welch, A.J.; Wissler, E.H.; Priebe, L.A. Significance of Blood Flow in Calculations of Temperature in Laser Irradiated Tissue. IEEE Trans. Biomed. Eng. 1980, BME-27, 164–166.
- Ramasamy, M.; Lee, S.S.; Yi, D.K.; Kim, K. Magnetic, Optical Gold Nanorods for Recyclable Photothermal Ablation of Bacteria. J. Mater. Chem. B 2013, 2, 981–988.
- Millenbaugh, N.J.; Baskin, J.B.; DeSilva, M.N.; Elliott, W.R.; Glickman, R.D. Photothermal Killing of Staphylococcus Aureus Using Antibody-Targeted Gold Nanoparticles. Int. J. Nanomed. 2015, 10, 1953–1960.
- Mocan, L.; Tabaran, F.A.; Mocan, T.; Pop, T.; Moşteanu, O.; Agoşton-Coldea, L.; Matea, C.T.; Gonciar, D.; Zdrehus, C.; Iancu, C. Laser Thermal Ablation of Multidrug-Resistant Bacteria Using Functionalized Gold Nanoparticles. Int. J. Nanomed. 2017, 12, 2255–2263.
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Nano-Photothermal Ablation Effect of Hydrophilic and Hydrophobic Functionalized Gold Nanorods on Staphylococcus Aureus and Propionibacterium Acnes. Sci. Rep. 2018, 8, 6881.
- Luo, J.; Deng, W.; Yang, F.; Wu, Z.; Huang, M.; Gu, M. Gold Nanoparticles Decorated Graphene Oxide/Nanocellulose Paper for NIR Laser-Induced Photothermal Ablation of Pathogenic Bacteria. Carbohydr. Polym. 2018, 198, 206–214.
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-Dependent Antibacterial Effects of Non-Cytotoxic Gold Nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468.
- Yakunin, A.; Zarkov, S.V.; Avetisyan, Y.A.; Akchurin, G.G., Jr.; Tuchina, E.S.; Tuchin, V. Modeling of Hyperthermia Induced by Functionalized Gold Nanorods Bound to Staphylococcus Aureus under NIR Laser Radiation. In Proceedings of the Saratov Fall Meeting 2018: Optical and Nano-Technologies for Biology and Medicine, Saratov, Russia, 24–28 September 2018; Volume 11065, pp. 279–288.
- Tuchin, V.V.; Popp, J.; Zakharov, V. Multimodal Optical Diagnostics of Cancer; Springer: Berlin/Heidelberg, Germany, 2020.
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective Laser Photo-Thermal Therapy of Epithelial Carcinoma Using Anti-EGFR Antibody Conjugated Gold Nanoparticles. Cancer Lett. 2006, 239, 129–135.
- Manuchehrabadi, N.; Chen, Y.; Lebrun, A.; Ma, R.; Zhu, L. Computational Simulation of Temperature Elevations in Tumors Using Monte Carlo Method and Comparison to Experimental Measurements in Laser Photothermal Therapy. J. Biomech. Eng. 2013, 135, 121007.
- Youn, J.-I. The Effect of an Optical Clearing Agent on Tissue Prior to 1064-nm Laser Therapy. Med. Lasers 2021, 10, 146–152.
- Chu, Y.; Liao, S.; Liao, H.; Lu, Y.; Geng, X.; Wu, D.; Pei, J.; Wang, Y. Second Near-Infrared Photothermal Therapy with Superior Penetrability through Skin Tissues. CCS Chem. 2021, 3, 3289–3300.
- Jia, H.; Chen, B.; Li, D. Dynamic Optical Absorption Characteristics of Blood after Slow and Fast Heating. Lasers Med. Sci. 2017, 32, 513–525.
- Yaroslavsky, A.N.; Schulze, P.C.; Yaroslavsky, I.V.; Schober, R.; Ulrich, F.; Schwarzmaier, H.-J. Optical Properties of Selected Native and Coagulated Human Brain Tissues in Vitro in the Visible and Near Infrared Spectral Range. Phys. Med. Biol. 2002, 47, 2059–2073.
- Yu, T.; Qi, Y.; Zhu, J.; Xu, J.; Gong, H.; Luo, Q.; Zhu, D. Elevated-Temperature-Induced Acceleration of PACT Clearing Process of Mouse Brain Tissue. Sci. Rep. 2017, 7, 38848.
- Laufer, J.; Simpson, R.; Kohl, M.; Essenpreis, M.; Cope, M. Effect of Temperature on the Optical Properties of ex Vivo Human Dermis and Subdermis. Phys. Med. Biol. 1998, 43, 2479–2489.
- Iorizzo, T.W.; Jermain, P.R.; Salomatina, E.; Muzikansky, A.; Yaroslavsky, A.N. Temperature Induced Changes in the Optical Properties of Skin in Vivo. Sci. Rep. 2021, 11, 1–9.
- Skinner, M.G.; Everts, S.; Reid, A.D.; Vitkin, A.; Lilge, L.; Sherar, M.D. Changes in Optical Properties Ofex Vivorat Prostate due to Heating. Phys. Med. Biol. 2000, 45, 1375–1386.
- Nagarajan, V.K.; Gogineni, V.R.; White, S.B.; Yu, B. Real Time Evaluation of Tissue Optical Properties during Thermal Ablation of ex Vivo Liver Tissues. Int. J. Hyperth. 2018, 35, 176–182.
- Nagarajan, V.K.; Ward, J.M.; Yu, B. Association of Liver Tissue Optical Properties and Thermal Damage. Lasers Surg. Med. 2020, 52, 779–787.
- Genin, V.D.; Bucharskaya, A.B.; Navolokin, N.A.; Terentyuk, G.S.; Khlebtsov, N.G.; Tuchin, V.V.; Genina, E.A. Impact of Immersion Agents on Optical Parameters of Tissues in the Process of Laser Photothermal Tumor Therapy: A Pilot Study. Opt. Spectrosc. 2022. in print.
- Bian, W.; Wang, Y.; Pan, Z.; Chen, N.; Li, X.; Wong, W.-L.; Liu, X.; He, Y.; Zhang, K.; Lu, Y.-J. Review of Functionalized Nanomaterials for Photothermal Therapy of Cancers. ACS Appl. Nano Mater. 2021, 4, 11353–11385.
- Yuan, M.; Xu, S.; Zhang, Q.; Zhao, B.; Feng, B.; Ji, K.; Yu, L.; Chen, W.; Hou, M.; Xu, Y.; et al. Bicompatible Porous Co3O4 Nanoplates with Intrinsic Tumor Metastasis Inhibition for Multimodal Imaging and DNA Damage–Mediated Tumor Synergetic Photothermal/Photodynamic Therapy. Chem. Eng. J. 2020, 394, 124874.
- Zhao, T.; Shen, X.; Li, L.; Guan, Z.; Gao, N.; Yuan, P.; Yao, S.Q.; Xu, Q.-H.; Xu, G.Q. Gold Nanorods as Dual Photo-Sensitizing and Imaging Agents for Two-Photon Photodynamic Therapy. Nanoscale 2012, 4, 7712–7719.
- Jiang, C.; Zhao, T.; Yuan, P.; Gao, N.; Pan, Y.; Guan, Z.; Zhou, N.; Xu, Q.-H. Two-Photon Induced Photoluminescence and Singlet Oxygen Generation from Aggregated Gold Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4972–4977.
- Vankayala, R.; Huang, Y.-K.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. First Demonstration of Gold Nanorods-Mediated Photodynamic Therapeutic Destruction of Tumors via Near Infra-Red Light Activation. Small 2013, 10, 1612–1622.
- Vankayala, R.; Kuo, C.-L.; Sagadevan, A.; Chen, P.-H.; Chiang, C.-S.; Hwang, K.C. Morphology Dependent Photosensitization and Formation of Singlet Oxygen (1Δg) by Gold and Silver Nanoparticles and Its Application in Cancer Treatment. J. Mater. Chem. B 2013, 1, 4379–4387.
- Vankayala, R.; Lin, C.-C.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. Gold Nanoshells-Mediated Bimodal Photodynamic and Photothermal Cancer Treatment Using Ultra-Low Doses of Near Infra-Red Light. Biomaterials 2014, 35, 5527–5538.
- Lv, J.; Zhang, X.; Li, N.; Wang, B.; He, S. Absorption-Dependent Generation of Singlet Oxygen from Gold Bipyramids Excited under Low Power Density. RSC Adv. 2015, 5, 81897–81904.
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332.
- Dahl, O. Interaction of Hyperthermia and Chemotherapy. In Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 1988; Volume 107, pp. 157–169.
- Urano, M. Invited Review: For the Clinical Application of Thermochemotherapy Given at Mild Temperatures. Int. J. Hyperth. 1999, 15, 79–107.
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and Adaptive Immune Cells in the Tumor Microenvironment. Nat. Immunol. 2013, 14, 1014–1022.
- Turubanova, V.D.; Mishchenko, T.A.; Balalaeva, I.V.; Efimova, I.; Peskova, N.N.; Klapshina, L.G.; Lermontova, S.A.; Bachert, C.; Krysko, O.; Vedunova, M.V.; et al. Novel Porphyrazine-Based Photodynamic Anti-Cancer Therapy Induces Immunogenic Cell Death. Sci. Rep. 2021, 11, 1–13.
- Liu, Y.; Chongsathidkiet, P.; Crawford, B.M.; Odion, R.; Dechant, C.A.; Kemeny, H.R.; Cui, X.; Maccarini, P.F.; Lascola, C.D.; Fecci, P.E.; et al. Plasmonic Gold Nanostar-Mediated Photothermal Immunotherapy for Brain Tumor Ablation and Immunologic Memory. Immunotherapy 2019, 11, 1293–1302.
- Zhang, Y.; Song, T.; Feng, T.; Wan, Y.; Blum, N.T.; Liu, C.; Zheng, C.; Zhao, Z.; Jiang, T.; Wang, J.; et al. Plasmonic Modulation of Gold Nanotheranostics for Targeted NIR-II Photothermal-Augmented Immunotherapy. Nano Today 2020, 35, 100987.
- Liu, Y.; Chorniak, E.; Odion, R.; Etienne, W.; Nair, S.K.; Maccarini, P.; Palmer, G.M.; Inman, B.A.; Vo-Dinh, T. Plasmonic Gold Nanostars for Synergistic Photoimmunotherapy to Treat Cancer. Nanophotonics 2021, 10, 3295–3302.
- Jung, B.-K.; Lee, Y.K.; Hong, J.; Ghandehari, H.; Yun, C.-O. Mild Hyperthermia Induced by Gold Nanorod-Mediated Plasmonic Photothermal Therapy Enhances Transduction and Replication of Oncolytic Adenoviral Gene Delivery. ACS Nano 2016, 10, 10533–10543.
- Chen, G.; Ding, L.; Wu, P.; Zhou, Y.; Sun, M.; Wang, K.; Oupický, D. Polymeric Micelleplexes for Improved Photothermal Endosomal Escape and Delivery of siRNA. Polym. Adv. Technol. 2018, 29, 2593–2600.
- Chong, L.M.; Tng, D.J.H.; Tan, L.L.Y.; Chua, M.L.K.; Zhang, Y. Recent Advances in Radiation Therapy and Photodynamic Therapy. Appl. Phys. Rev. 2021, 8, 041322.
- Cai, R.; Xiang, H.; Yang, D.; Lin, K.-T.; Wu, Y.; Zhou, R.; Gu, Z.; Yan, L.; Zhao, Y.; Tan, W. Plasmonic Heterostructure with Enhanced Synergistic Efficacy for Radiophotothermal Therapy. J. Am. Chem. Soc. 2021, 143, 16113–16127.
- Kayani, Z.; Islami, N.; Behzadpour, N.; Zahraie, N.; Imanlou, S.; Tamaddon, P.; Salehi, F.; Daneshvar, F.; Perota, G.; Sorati, E.; et al. Combating Cancer by Utilizing Noble Metallic Nanostructures in Combination with Laser Photothermal and X-Ray Radiotherapy. J. Drug Deliv. Sci. Technol. 2021, 65, 102689.
This entry is offline, you can click here to edit this entry!
