Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Alcohol consumption is linked to the risk of prostate cancer (PCa). High alcohol intake, especially binge drinking, is associated with increased risk for PCa, and this effect is not limited to any type of beverage. Alcohol consumption is also directly linked to PCa lethality as it may accelerate the growth of prostate tumors and significantly shorten the time for the progression to metastatic PCa.
- alcohol consumption
- prostate cancer
- prostate cancer-associated mortality
1. Introduction
The link between alcohol consumption and malignant diseases has long intrigued researchers. In a review of 140 references from 1966 to 2020, mainly epidemiological studies, moderate alcohol consumption was correlated with increased risk of upper digestive tract, liver, colorectal, breast, pancreatic, and prostate cancers (PCa) [1]. Despite generally increased cancer risk from any type of alcohol, many patients continue to drink alcohol [2], and some governments continue to leave their citizens uninformed of the carcinogenic risk of alcohol consumption [3]. In a recent report, the World Health Organization (WHO) estimated that alcohol use resulted in 3 million deaths globally in 2016, 12.6% of those were associated with malignant neoplasms [4]. Among men, alcohol intake is the fourth-largest contributor to cancer [5].
In 2019, 25.8% of Americans ages 18 or older reported that they engaged in binge drinking in the past month, and another 6.3% reported that they consumed alcohol at a heavy level in the past month [6]. According to the 2019 National Survey on Drug Use and Health (NSDUH), 14.1 million US adults ages 18 and older (5.6% of this age group) had Alcohol Use Disorder (AUD) [7]. This includes 8.9 million men (7.3% of men in this age group) and 5.2 million women (4.0% of women in this age group). Among PCa patients from the 2012–2017 National Health Interview Survey (NHIS), the distribution of alcohol consumption was as follows: 4.2%—heavy drinkers (>14 drinks per week), 47.1%—light/moderate drinkers (up to 14 drinks per week), 12.2%—infrequent drinkers (1–11 drinks in the past year), 25.7%—former drinkers (≥12 drinks in a lifetime but with 0 drinks in the past year), and 10.8%—never drinkers (<12 drinks in a lifetime) [8].
2. The Impact of Alcohol on the Aggressiveness of Prostate Tumors and PCa-Associated Death
Chronic alcohol consumption has been shown to induce high-grade PCa and metastasis [114,115,116,117,118,119]. One US-based study found that a higher frequency of alcohol intake was associated with increased mortality from PCa [120]. In another survey, Canadian researchers interviewed PCa patients immediately after diagnosis and 2–3 years later to monitor the level of alcohol consumption; mortality data were collected for up to 19 years [119]. They found that patients who consumed more than eight drinks weekly had higher mortality than patients who stopped drinking after being diagnosed with PCa.
Observations from Australian patients indicate that a beer intake frequency of ≥5 days per week was associated with an increased risk of advanced PCa (histological Gleason score eight or higher) [121]. Similarly, an extensive analysis among the Japanese population found a positive association of alcohol consumption with PCa in subjects with advanced disease: compared to non-drinkers, increased risks were observed for those who consumed 0–149 g/week, 150–299 g/week, and ≥300 g/week [122].
A recent study aimed to analyze the link between the total intake of alcohol and death from cancer. Using a large international dataset from the United Nations (UN), Food and Agriculture Organization of the United Nations (FAO), Organization for Economic Co-operation and Development (OECD), World Bank, WHO, U.S. Department of Agriculture, U.S. Department of Health, and Eurobarometer, authors came to the unequivocal conclusion that alcohol is “significantly and positively associated with prevalence and mortality from total, colon, lung, breast, and prostate cancers” [123].
Compared to men without early-life use of alcohol, military veterans with heavier alcohol consumption earlier in life were more likely to be diagnosed with high-grade PCa [124]. Recent US-based analysis also suggested that states with more restrictive alcohol policies had lower alcohol-attributable PCa mortality [125]. Accordingly, the authors stated that “strengthening alcohol control policies may be a promising cancer prevention strategy”. These conclusions are reflected in another US-based study which stated that reducing alcohol intake below one drink per day is associated with a significant decrease in risk of PCa-related death [126]. Similarly, an investigation from Japan found PCa-related death among daily drinkers increased 2.5 times [76].
Significantly, PCa mortality among Mormons and Seventh-day Adventists from Germany, who usually consumed alcohol at a low level, is reduced considerably compared with PCa patients from the best German cancer registry (Saarland) [127]. Interestingly, countries with the lowest mortality rate for PCa (Bhutan, Nepal, Bangladesh, North Korea, Turkmenistan, Uzbekistan, Sri Lanka, Tajikistan, and Yemen [128]) are also characterized by the lowest levels of alcohol consumption [129]. Another recent study analyzed PCa mortality data obtained from 71 countries through the International Agency for Research on Cancer and the Food and Agricultural Organization of the United Nations. Authors found a correlation between increased cases of PCa-related deaths and total animal fat calories, meat, milk, sugar, alcoholic beverages, and stimulants [130].
A recent Mendelian randomization study from the international PRACTICAL Consortium found that SNP within the ALDH1B1 gene was associated with mortality in men with low-grade PCa [32]. The authors of this manuscript concluded: “Reducing alcohol consumption could slow prostate cancer disease progression.” Another study of 3306 PCa patients with European ancestry from the PCa Consortium aimed to examine alcohol intake and SNPs in the four pathways associated with PCa aggressiveness (angiogenesis, mitochondria, miRNA, and androgen metabolism-related pathways) [131]. Investigators found that excessive alcohol intake significantly impacts PCa aggressiveness within the following genetic subgroups: CAMK2D (calcium/calmodulin-dependent protein kinase II delta, enriched in the cell cycle and the calcium signaling pathway), PRKCA (protein kinase C alpha, the modulator of cell adhesion and transformation), and ROBO1 (roundabout guidance receptor 1, cell migration factor and a tumor suppressor gene). In addition, the authors observed an additional moderate link between alcohol and SNPs of genes associated with PCa aggressiveness. These are HGF (hepatocyte growth factor), PDGFB (platelet-derived growth factor subunit B), SYK (spleen-associated tyrosine kinase), PDGFD (platelet-derived growth factor D), and COL4A3 (collagen type IV alpha 3 chain) [131].
It has been shown that SNPs in the TNF-α and IL-10 genes were associated with an increased PCa risk [132,133]. A recent study from the Indian population (105 PCa cases along with 115 control) found that an increasing percentage of TNF-α and IL-10 haplotypes were found to be positively associated with aggressiveness of PCa and alcohol consumption [134].
3. The Cellular Mechanisms of Alcohol’s Effect on the Progression of PCa
In recent years, it has become clear that alcohol has multiple damaging effects on intracellular organization, transportation, and structure of organelles [178]. Both acute and chronic alcohol exposure results in disorganization of Golgi and inhibition of both intra-Golgi and Golgi-to-plasma membrane trafficking. This, in turn, leads to intracellular accumulation of newly synthesized proteins and subsequent ER stress [179,180,181,182,183,184,185]. In addition, EtOH and its metabolites alter the activity of different Rab proteins, the conductors of post-Golgi transportation, and endocytosis [186,187,188,189,190,191,192,193,194]. Our laboratory revealed that alcohol-induced Golgi disorganization is associated with impairment of both coat protein I (COPI) and coat protein II (COPII) vesiculation and monomerization of the Golgi scaffold proteins, golgins [180,195]. Dimerization of giantin, the largest golgin, appears to be essential for the post-alcohol recovery of Golgi in hepatocytes [196]. Interestingly, giantin also loses its dimeric conformation in advanced PCa cells, which is associated with severe Golgi fragmentation and translocation of critical Golgi glycosyltransferases from the Golgi to the ER [197,198,199]. Recent investigations indicated that Golgi disorganization is a hallmark of cancer progression [200,201,202,203]. We introduced the concept of an “onco-Golgi”, which postulates that in different types of cancer, including PCa, the activation of many pro-oncogenic and pro-metastatic pathways is caused by the mislocalization of resident Golgi enzymes due to disorganization of this organelle [204].
We recently reported that in androgen-responsive PCa cells exposed to EtOH, Golgi disorganization is triggered by ACH and results in enhanced anchorage-independent growth, adherence, migration, and secretion of PSA [205]. Alteration of the Golgi morphology leads to the translocation of glycogen synthase kinase β (GSK3β) from the Golgi to the cytoplasm, followed by the phosphorylation of histone deacetylase 6 (HDAC6) and activation of the downstream heat shock protein 90 (HSP90)–androgen receptor (AR) pathway [205] (Figure 4A). Therefore, EtOH may accelerate AR transactivation, the driver of prostate carcinogenesis. Significantly, EtOH administration promoted the growth of LNCaP-derived xenograft tumors in mice.
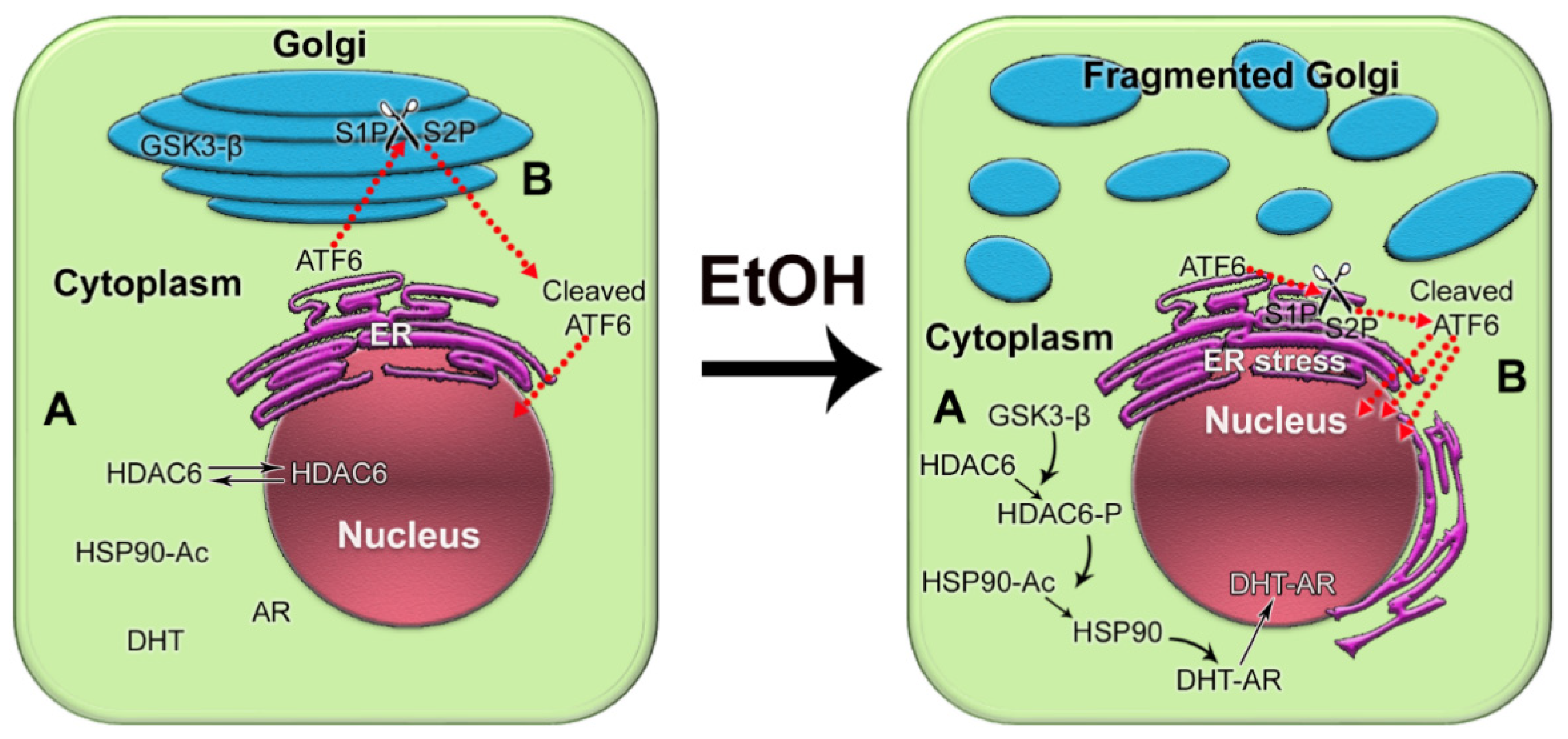
Figure 4. The impact of alcohol-induced Golgi disorganization on prostate carcinogenesis. (A) In normal prostate cells or low aggressive PCa cells, HDAC6 is distributed in both the nucleus and the cytoplasm. Typically, the phosphorylation of HDAC6 is moderate because the enzyme that phosphorylates HDAC6, GSK3β, is sequestered primarily within the Golgi. The acetylated HSP90 has a limited binding capacity to AR. EtOH treatment results in Golgi fragmentation and translocation of GSK3β to the cytoplasm, which results in increased phosphorylation of HDAC6. HDAC6-P deacetylates HSP90, which, in turn, accelerates conformational maturation of AR, its binding to DHT, and translocation to the nucleus. (B) In normal prostate and low-aggressive PCa cells, ATF6α is cleaved sequentially in the Golgi by S1P and S2P proteases. The dimeric form of trans-golgin GCC185 is the retention partner for both S1P and S2P. Cleaved ATF6 enters the nucleus and binds to ER stress-response elements, stimulating the expression of UPR genes. EtOH and its metabolites fragment Golgi membranes, which is associated with the monomerization of GCC185 and the subsequent shift of S1P and S2P to the ER. This simplifies and accelerates ATF6 cleavage, resulting in more prominent UPR signaling to maintain tumor cell growth and proliferation.
Next, we observed that the effect of alcohol on Golgi in low passage androgen-responsive LNCaP cells mimicked the fragmented Golgi phenotype of androgen-refractory high passage LNCaP and PC-3 cells [206]. Transition to androgen unresponsiveness was accompanied by the downregulation of N-acetylglucosaminyltransferase-III (MGAT3), the enzyme that competes with N-acetylglucosaminyltransferase-V (MGAT5) for anti-metastatic N-glycan branching. Moreover, in low passage LNCaP cells, alcohol-induced Golgi fragmentation resulted in translocation of MGAT3 from the Golgi to the cytoplasm, while intra-Golgi localization of MGAT5 appeared unaffected. We observed that within the same clinical stage, the level of Golgi fragmentation and the shift of MGAT3 from Golgi to the ER were more prominent in alcohol-consuming patients [206].
Alcohol’s tumor-promoting effect is also mediated via ER stress. It is known that neoplastic transformations are frequently accompanied by calcium deprivation, oxidative stress, aerobic glycolysis, and DNA damage [207,208,209]. These factors trigger ER stress and launch the unfolded protein response (UPR) [210], aiming to enhance the proliferation of cancer cells and maintain their metabolic homeostasis. The latter, in turn, should adjust the tumor microenvironment to facilitate its survival and expansion [211]. Moreover, angiogenesis and production of antiapoptotic factors and pro-inflammatory cytokines are largely ascribed to ER stress and UPR [212,213,214]. Several recent studies have implicated ER stress and UPR in the development of PCa and the progression of castration-resistant prostate cancer (CRPC) [215,216].
Considering the facts described above, we recently investigated the link between alcohol-induced Golgi disorganization and one of the UPR branches, activating transcription factor 6 (ATF6), which is known to be activated in PCa cells [215,216,217,218]. Typically, 90 kDa ATF6 translocates to the Golgi to be cleaved by site-1 protease (S1P) and site-2 protease (S2P), releasing the 50 kDa fragment. The cleaved ATF6 moves to the nucleus, where it initiates transcription of the genes involved in the resistance by ER stress and UPR [219]. We utilized in vitro and in vivo models of chronic alcohol consumption. In addition, we analyzed tissue sections from normal prostate and from the PCa patients categories with the same grade and Gleason score: non-drinking patients (who do not drink or drink less than once per month) and patients who regularly consume alcohol at a moderate level (12 oz. beer—5–6 times per week; 3–5 glasses of wine per week; 3.4 oz. of strong liquor—2–3 times per week) or at a heavy level (12 oz. beer—2 or more times per day; 4 oz. glass of wine—1 or more times per day; 3.4 oz. of strong liquor at least once per day) [220]. We found that alcohol-induced Golgi disorganization was associated with monomerization of trans-golgin, GCC185, the Golgi retention partner of S1P and S2P. This leads to translocation of S1P and S2P from Golgi to ER, followed by intra-ER cleavage of ATF6, accelerated UPR, and cell proliferation (Figure 4B). The segregation of S1P and S2P from Golgi and activation of ATF6 are positively correlated with AR signaling, different disease stages, and alcohol consumption. Significantly, the depletion of ATF6 retarded the growth of xenograft prostate tumors and blocked the production of pro-metastatic metabolites [220]. Another important observation from our study is that in PCa patients consuming alcohol, cribriform patterns in the tumor area (a clinically significant prognostic indicator for advanced PCa) were larger than in non-alcoholic patients. The intranuclear signal of ATF6 in these patterns was enhanced in patients drinking at a high level compared with that in the control group or among moderately drinking patients.
Recent observation indicates that, in the TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model of PCa, chronic EtOH administration (10% in saline) accelerates the tumor growth and metastasis [221]. In primary prostate tumors, alcohol-treated mice, compared to the control, demonstrate significantly increased expression of nuclear factor kappa B (NF-κB), supporting a proinflammatory effect of EtOH. In addition, matrix metallopeptidase (MMP2), laminin, integrin β1, and fibronectin were also elevated, suggesting the enhanced metastatic potential of EtOH-treated tumor cells. Interestingly, co-administration of EtOH and NF-κB inhibitor parthenolide prevent such effects of alcohol, suggesting the leading role of the NF-κB-mediated pathway in alcohol-associated tumor promotion.
Overall, the results presented above indicate that multiple pathways are involved in the underlying mechanisms of alcohol’s effect on prostate carcinogenesis. However, at this point, there is no clear understanding of how EtOH accelerates the development of CRPC, where the tumor no longer completely responds to androgen deprivation therapy (ADT).
4. Conclusions
Despite the limitations discussed here, the link between alcohol consumption and the development of PCa is strong. Still, PCa development depends critically on other factors, notably diet, smoking, age, race (black men have higher incidence and mortality than white men), physical and sexual activity, stress, obesity, family history of PCa, and chronic prostatitis [229,230,231] (Figure 5A). For instance, the risk of PCa associated with the pro-inflammatory potential of the diet is accelerated in low-to-moderate alcohol drinkers [232]. In addition, alcohol intake was directly associated with PCa risk among individuals with lower dietary fiber intake and low folate intake [233,234].
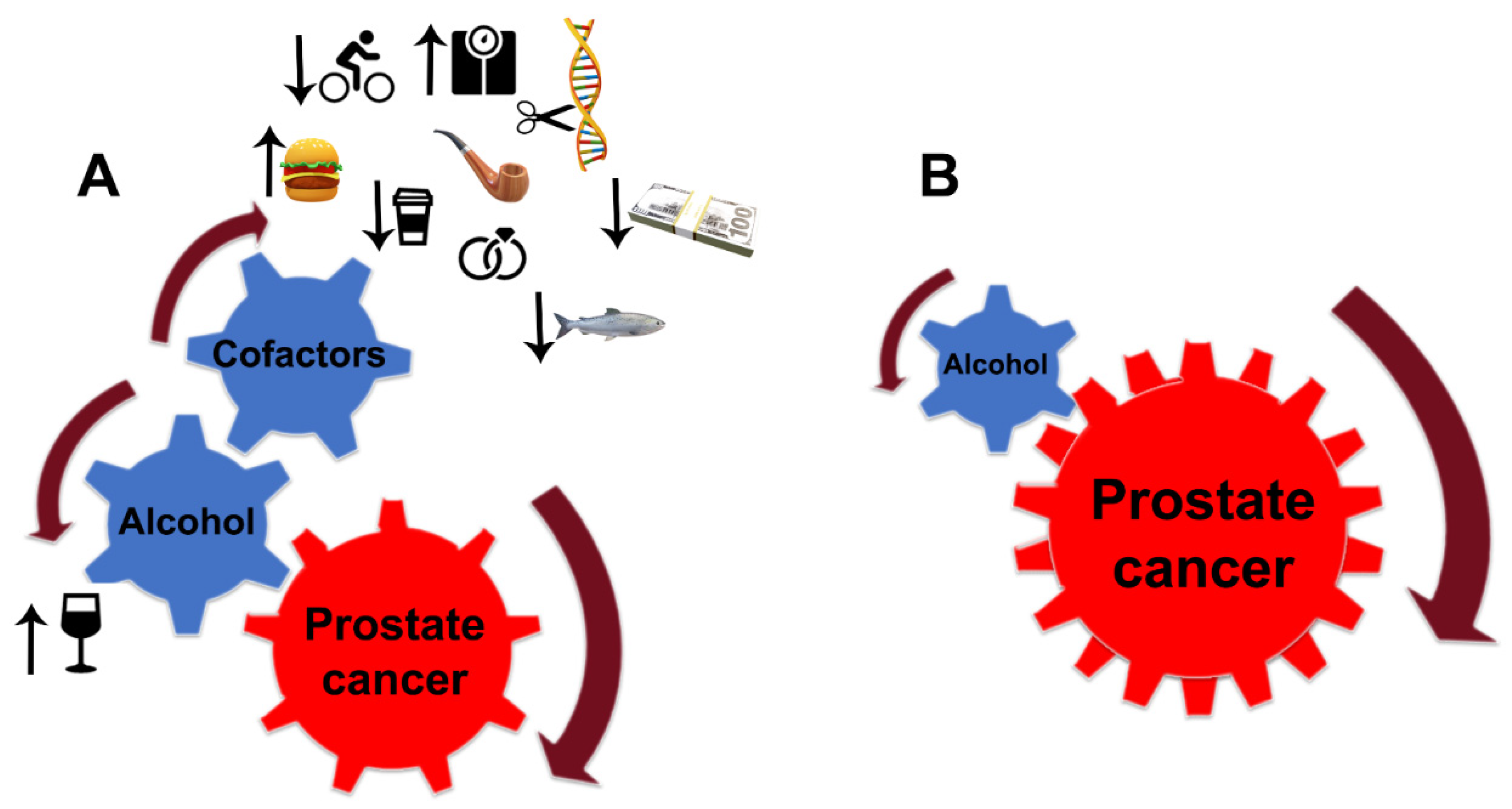
Figure 5. Alcohol interference in the development and progression of PCa. (A) Alcohol is a critical player and driver of prostate carcinogenesis. The carcinogenic effects of EtOH and its metabolites are magnified by multiple cofactors, such as obesity, smoking, excessive high-fat and red meat diet, low-level consumption of fish, caffeine, and linoleic acid, low physical activity, SNP of alcohol-related genes, and family history of PCa. Additional factors may include income and marital status: unmarried patients with an unstable financial situation are at higher risk of PCa. (B) In patients diagnosed with PCa, alcohol’s contribution to prostate tumor progression does not require cofactors. EtOH metabolites are sufficient to drive tumor growth and raise the metastatic potential of cancer cells.
Next, dietary preferences may cardinally vary according to geographical area. For instance, the Mediterranean diet (high intake of vegetables, legumes, fresh fruit, non-refined cereals, nuts, and olive oil, with moderate consumption of fish and dairy, low intake of red meats, and infrequent use of red wine in low dosage) was associated with a low incidence of PCa and low mortality rate in patients without metastasis [235,236,237].
PCa risk is positively correlated with the number of drinks and frequent episodes of binge drinking. Individuals who have first-degree family members with PCa should consider moderate, infrequent alcohol use. Patients diagnosed with any stage of PCa should consider quitting drinking since, even at a moderate level of consumption, EtOH and its metabolites alone are enough to accelerate tumor growth and enhance the metastatic potential of cancer cells. Under such circumstances, the contribution of other risk factors is negligible (Figure 5B). The same strategy should be employed for PCa patients after prostatectomy.
Several factors may be responsible for the noted discrepancies between studies that showed a positive link between alcohol and PCa risk and those that failed to find such an association. These include varied sample sizes, types of alcohol considered, criteria used for control selection and alcohol history categorization, diet, and inaccurate self-reporting. Studies with a larger sample size allowed for greater statistical power but require a similar increase in complexity to adequately control for the variables discussed here. Moreover, it is critical to evaluate the impact of alcohol in patients having a long history of alcohol consumption, as different studies found that lifetime, but not current, alcohol intake is positively correlated with the probability of PCa development [78,79,234,238]; an increasing number of drinking years increased the risk of PCa [239]. For instance, when a study in Brazil was conducted based on lifetime drinking, a positive link between alcohol and PCa was detected [91]. However, in the observation among Brazilian patients with current drinking status only, the risk of PCa was slightly reduced [102].
Epidemiological studies that aim to investigate the risk of PCa among alcohol-consuming patients cannot consider all contributing factors. The most popular variables matched for the cases and controls in case-control studies were age, race and residency, poverty census enumeration district, family income, tea and coffee consumption, serum vitamin A level, education, physical activity, body mass index, smoking status, marital status, dietary preferences, family history of cancer, use of PSA screening, total lifetime female sexual partners, family income, age of diagnosis, height, total energy, carbohydrates, and linoleic acid [240]. Unfortunately, some studies did not include important exclusions for control groups, such as history of any other neoplasm, prostatectomy, and presence of prostatic diseases confirmed by transrectal ultrasonography or digital examination. This limits the statistical power to detect significant correlations between control and PCa groups. The ideal control group would be those screened for PCa but not histologically confirmed.
Many observations did not consider the sick-quitter effect. There are always patients considering themselves non-drinkers because they have previously quit drinking alcohol due to a non-cancer-related condition. Additionally, as with all studies that rely on self-reporting by participants, misreporting (either intentionally or inadvertently) of the level of alcohol consumption cannot be avoided. There is a chance that high consumers were falsely categorized as moderate consumers, leading to an underestimation of the risks. Misunderstanding of the “standard drink” may also contribute to misreporting. The standard drink (or one alcoholic drink equivalent), according to US National Institute on Alcohol Abuse and Alcoholism, contains roughly 14 g of pure EtOH, which is found in 12 ounces (~355 mL) of regular beer, which is usually about 5% alcohol; 5 ounces (~150 mL) of wine, which is typically about 12% alcohol; 1.5 ounces (~45 mL) of distilled spirits, which is about 40% alcohol. However, retrospective consideration is prone to recall bias. In many cases, especially social drinking, patients can not precisely count the actual amount of alcohol consumed within a week. Therefore, the number of drinks per week may vary within studies, depending on the subjective calculations of patients, which, in turn, may affect the accuracy of statistical analysis in both control and PCa groups.
Extensive international studies are required to evaluate the impact of specific beverages on PCa development, lest we miss the forest for the trees. In particular, there is insufficient evidence to conclude that moderate drinking of wine (either red or white) could slow PCa growth or metastasis. The potential antioxidant effect of wine’s polyphenols can be outweighed by the tumor-promoting mechanisms of EtOH described here. In the meantime, epidemiological data suggest that individuals consuming wine in moderation are at low risk for BPH.
Lastly, serum PSA data should be cautiously evaluated for patients regularly drinking alcohol at a high level because it can be affected by alcohol intake immediately prior to testing and by a general history of alcohol consumption. Additionally, the observed level of male sex hormones is irrelevant to the alcoholism-associated risk and progression of PCa.
This entry is adapted from the peer-reviewed paper 10.3390/biom12030375
This entry is offline, you can click here to edit this entry!
