Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Virology
|
Biochemistry & Molecular Biology
Genes encoding ejection proteins are commonly found in Podoviridae phages that infect Gram-negative bacteria, including Enterobacteriaceae, Mycobacteria, Pseudomonadaceae, and Cyanobacteria.
- ejection proteins
- DNA ejectosome
- viral genome ejection
- cell envelope
- bacteriophage T7
- internal core proteins
- gp14
- gp15
- gp16
1. Conservation of Ejection Proteins
Genes encoding ejection proteins are commonly found in Podoviridae phages that infect Gram-negative bacteria, including Enterobacteriaceae, Mycobacteria, Pseudomonadaceae, and Cyanobacteria. However, they are not found in phi29-like phages, which are also members of the Podoviridae family that infect Gram-positive bacteria and that have a completely different cell envelope consisting of only one lipid membrane and a thicker peptidoglycan layer. Using phage-T7 ejection proteins as a reference, it was bioinformatically identified the genes encoding the homologous gp14, gp15, and gp16 proteins in fourteen Podoviridae family members that infect Escherichia coli (T7, CUS-3, 13a, BA14, K1E, HK620), Salmonella (P22, Epsilon15, SP6), Shigella (Sf6), Prochlorococcus (P-SSP7), Klebsiella (K11), Yersinia (phiYeO3-12), and Pseudomonas (phiKMV) (Table 1). The ejection-protein genes are clustered in a small operon, where the gene encoding the gp14-like factor is adjacent to gp15, followed by a larger ORF encoding gp16. It was found that a marked divergence in the size of gp15 and gp16 versus gp14. Gp15 can vary by as much as 138% among different phages, from 431 aa in Sf6 to 982 aa in phage K1E. Similarly, gp16 varies in size by as much as 120%, from 609 aa in P22 to 1337 aa in phage phiKMV. In contrast, gp14 is more consistent and varies in size by less than 30% (from 181 aa in phiKMV to 240 aa in K1E) (Table 1). In general, P22-like phages (for example, P22, Sf6, HK620, CUS-3) [1] appear to have a significantly smaller gp15 and gp16 than other Podoviridae. This is interesting considering that in P22-like phages, the ejection proteins do not form a core stack in the pre-ejection conformation [2] but are dispersed inside the virion, possibly residing in the proximity of the portal protein [3].
Table 1. List of gene factors homologous to T7 gp14, gp15 and gp16. Highlighted in bold are the largest and smallest representatives of each group. Annotations for gp15-like and gp16-like gene factors ranging from extra-small (XS) to extra-large (XL) included for comparison within each grouping.
| Phage (Host) | gp14-Like (OM) |
gp15-Like (Tunnel) |
gp16-Like (IM) |
|---|---|---|---|
| T7 (Escherichia coli) | gp14 (196 aa) | gp15 (747 aa) M | gp16 (1318 aa) XL |
| CUS-3 (Escherichia coli) | gp7 (230 aa) | gp20 (449 aa) S | gp16 (719 aa) S |
| 13a (Escherichia coli) | gp14 (196 aa) | gp15 (747 aa) M | gp16 (1318 aa) XL |
| BA14 (Escherichia coli) | gp14 (201 aa) | gp15 (759 aa) M | gp16 (1315 aa) XL |
| K1E (Escherichia coli) | gp34 (240 aa) | gp35 (982 aa) XL | gp36 (1102 aa) M |
| HK620 (Escherichia coli) | gp7 (230 aa) | gp20 (449 aa) S | gp16 (722 aa) S |
| P22 (Salmonella enterica) | gp7 (229 aa) | gp20 (471 aa) S | gp16 (609 aa) XS |
| Epsilon 15 (Salmonella enterica) | gp11 (229 aa) | gp12 (499 aa) S | gp13 (708 aa) S |
| SP6 (Salmonella enterica) | gp35 (239 aa) | gp36 (978 aa) XL | gp37 (1270 aa) L |
| Sf6 (Shigella flexneri) | gp11 (230 aa) | gp12 (431 aa) S | gp13 (665 aa) XS |
| P-SSP7 (Prochlorococcus marinus) | gp14 (200 aa) | gp15 (837 aa) L | gp16 (1245 aa) L |
| K11 (Klebsiella pneumoniae) | gp14 (196 aa) | gp15 (751 aa) M | gp16 (1321 aa) XL |
| phiYeO3-12 (Yersinia enterocolitica) | gp14 (197 aa) | gp15 (747 aa) M | gp16 (1320 aa) XL |
| phiKMV (Psudomonas aeruginosa) | gp35 (181 aa) | gp36 (898 aa) L | gp37 (1337 aa) XL |
Ejection-protein sequences were aligned with ClustalW [4] and converted to phylip format [1] for phylogenetic-tree calculation using PhyML 3.0 [5]. This analysis revealed that each ejection protein falls into at least three major groups, which have diverged greatly throughout evolution, in a neighbor-joining tree highlighted with different-colored boxes in Figure 2A–C. For ease of description, the three major groups are named by three representative phages: T7, P22, and SP6. Interestingly, the three ejection proteins are the most diverse between P22- and T7-like phages (for example, T7, 13a, BA14, phiYe03, K11), whereas the SP6 group was clustered with T7 phages for gp16 and P22 phages for gp14 and gp15 (Figure 2A–C). However, Epsilon15 is an out-group in the gp15 tree but clusters with P22 in the gp14 and gp16 trees. This phage has a ‘small’ gp15 and gp16 (Table 1), similar to P22-like phages, but also displays a core stack in the pre-ejection conformation, similar to phage T7 [6]. Below, it will briefly discuss the conservation of each ejection protein.
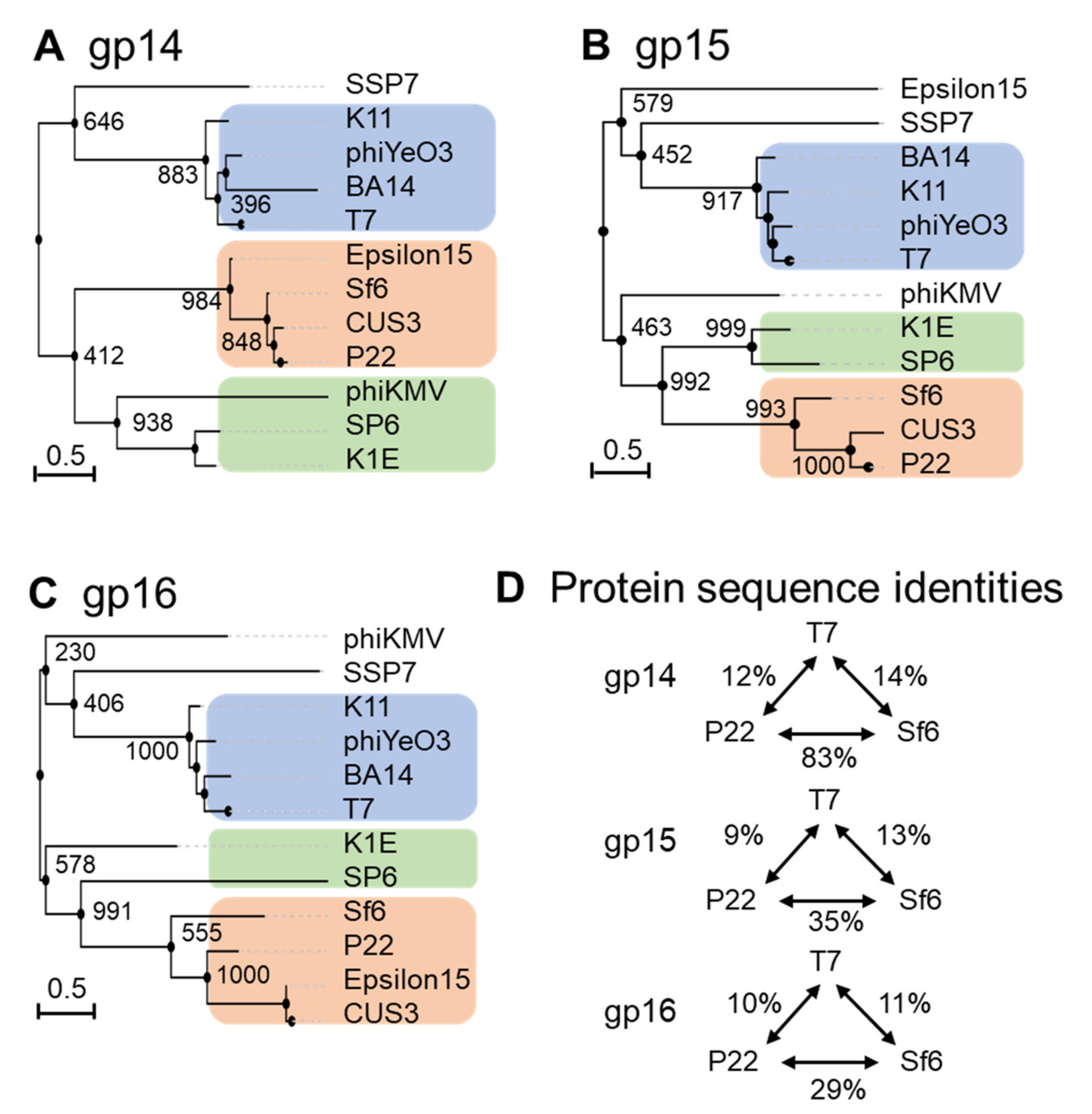
Figure 2. Diversity of the ejection proteins across fourteen representative Podoviridae. Phylogenetic trees for (A) gp14-like, (B) gp15-like and (C) gp16-like ejection proteins created using FastME 2.0 and PhyML 3.0 [5][7] with branch support of 1000 bootstrap repeats. (D) Table data on sequence identities and similarities with respect to the T7, P22 or SP6 ejection proteins were calculated using SIAS (http://imed.med.ucm.es/Tools/sias.html; accessed on 5 January 2022).
Gp14 is the most conserved of the three ejection proteins, both in sequence and size (about 200 aa), with an average sequence identity and similarity of 19/29% among the phages analyzed (Figure 2A). T7 phages appear to be more divergent than P22 and SP6 phages, sharing an average sequence identity of 12% and 8%, respectively (Figure 2D). All T7 gp14-like factors contain a set of four or five predicted transmembrane helices, except P-SSP7 gp14, which is predicted to have only two TMHs [8]. These predicted transmembrane α-helices likely allow all gp14 homologs to insert into the host OM, as also revealed by membrane-localization studies in T7 [9][10][11].
Gp15-like homologs vary significantly in size (between 431–982) (Table 1), with an overall sequence identity between 7% and 9% in the three groups identified in Figure 2B. Homology matching to T7 gp15 is challenging as most are matched based on size and synteny rather than demonstrating sequence similarity (Table 1 and Figure 2B) [12]. One study suggested rapid evolutionary divergence occurs for gp15-like proteins as their sequences have diverged to only share 34% identity across 0.4 billion years, whereas on the same time scale, portal protein homologs have retained 69% sequence identity (Figure 2B) [12][13]. Gp15-like homologs are better conserved in close T7 relatives, which have an average sequence identity and similarity of 19/39% (Table 1 and Figure 2B) and even more in P22 phages, with an average sequence identity and similarity of 36/55% (Figure 2B). Salmonella-phage P22 gp15-homolog gp20 (471 aa) is ejected upon adsorption on the host and extends the extra-cellular channel across the OM and into the periplasm (Table 1) [2]. Low-resolution structural data and biochemical evidence suggest that P22 gp20 forms a channel extending the tail complex while other ejection proteins span the envelope [2][14][15]. P22 gp20 may also require cleavage by a host enzyme before becoming functional, suggesting that it may be an exception to other homologs (Table 1 and Figure 2B) [14]. Purified Shigella-phage Sf6 gp12 (431 aa), which is more closely related to P22 gp20 than T7 gp15, forms a tube-like structure [16] (Figure 2B). Overall, it is likely that all gp15-like homologs are functionally similar, forming the same core tunnel based on consistent secondary-structural elements with some gaps and insertions among close T7 relatives. Surprisingly, some gp15-like homologs such as E. coli phages K1E [17] and K1-5 homolog gp35 contain lysozyme activity, which is found on gp16 in the T7 system (Table 1 and Figure 2B) [18][17]. The swapping of functional domains across ejection proteins is thematic for the pervasive mosaicism among related phages [15].
Gp16-like homologs also vary significantly in size (between 609–1337) and domain composition [12] (Table 1). T7-like gp16 homologs have an average sequence identity and similarity of 20/40%, which drops to 16/37% among P22-related phages, suggesting rapid divergence between and within these phage groups (Table 1 and Figure 2C). Interestingly, the T7 gp16 N-terminal peptidoglycan-hydrolase domain is not conserved in P22 or Sf6, where this ejection protein is significantly smaller (example, 1318 vs. 609 and 665, respectively, Table 1). However, the T7 gp16 putative transmembrane helices at the C-terminus [19][20] were identified in all gp16-like homologs presented in Table 1. Additionally, gp16’s positively charged five C-terminal residues that are necessary for infectivity are also conserved among T7’s closest relatives (Table 1 and Figure 2C) [10]. Salmonella-phages P22 and Epsilon15 gp16-like homologs show little sequence similarity to the T7 counterpart despite having matching synteny and implications in forming a tube for genome ejection (Table 1 and Figure 2C) [12][21]. The divergence of gp16 homologs in T7- and P22-like phages suggests the plastic evolution of this protein to solve the challenges of genome delivery [12]. Interestingly, a smaller gp16-C (as in P22-like phages) correlates with the loss of a core stack in the pre-ejection conformation. It was speculated that a small C-terminal domain reduces the stability of the gp16 tetramer in the head and its ability to form a defined quaternary structure. Interestingly, the existence of smaller gp15 and gp16 ejection proteins, which are not part of a core stack, as in P22-like phages, correlates with the existence of a portal-protein barrel [22][23]. Intriguingly, in all phages where the ejection proteins are organized into a core stack prior to ejection, the portal protein lacks a C-terminal barrel.
2 Models for Ejection-Protein Assembly into a DNA Ejectosome
The exact mechanisms by which T7 expels the ejection proteins and their assembly into a DNA ejectosome remains unknown. It was conceptualized recent structures of T7 ejection proteins in pre- and post-ejection conformations and previous biochemical data into two models that was named the “Inverted-Sock model” and the “Octopus model”.
The two models differ in the way the ejection proteins assemble into a transmembrane-envelope channel and are based on three assumptions. (i) In the pre-ejection conformation, the internal core is aligned on top of the portal/tail axis (Figure 3A) [24]. (ii) T7 tail-fiber interactions with the host LPS triggers conformational changes within the T7 tail, widening the nozzle to 30 Å, and signals the expulsion of ejection proteins gp14, gp15, and gp16 and the viral genome (Figure 3B) [25]. (iii) The first ejection protein to exit through the portal channel is gp14, which extends the nozzle and inserts into the OM, creating a hexameric, constitutively open pore (Figure 3B) [24][10]. In the “Inverted-Sock model,”it was hypothesized that gp16 exits through the portal-tail-gp14 complex channel as a monomer and is ejected into the periplasmic space where it cleaves peptidoglycan via its N-terminal peptidoglycan-hydrolytic domain (Figure 3C). Next, gp15 is expelled as a monomer into the periplasm where interactions with gp14 stabilize its flexible N-terminal domain [26] and interactions with the gp16-N molecular tape stabilize gp15’s flexible C-terminal domain resulting in the gp15:gp16-N hexameric periplasmic tunnel [8]. Expectedly, gp16-C inserts into the host IM, creating a transient pore and projecting a large gp16-C cytoplasmic hub for viral-genome translocation. The “Inverted-Sock model” is aptly named as the transition of ejection proteins from the core stack to the cell envelope mimics the movement of reaching into a sock and inverting it. The “foot” of the metaphorical sock (gp16), which is furthest away from the opening (portal-gp14 OM pore), is pulled through prior to the “tube” of the sock (gp15 forming the periplasmic tunnel tube). In the “Octopus model,” following gp14’s ejection and formation of the OM pore [24], it was hypothesized that gp15 exits by forming a stable N-terminal hexameric complex between gp14 in the OM pore and the peptidoglycan barrier with disordered C-terminal arms splayed like an octopus (Figure 3D). Via the portal-tail-gp14-gp15-N connected tunnel, it was presumed that gp16-N to egress and cleave through the peptidoglycan barrier with its transglycosylase activity. Thereafter, gp16-N molecular-tape residues stitch together the disordered gp15 C-terminal regions, assembling the hexameric periplasmic tunnel, which spans the entire periplasm. Gp16-C then breaches the host IM and projects the cytoplasmic hub for viral-genome translocation (Figure 3E).
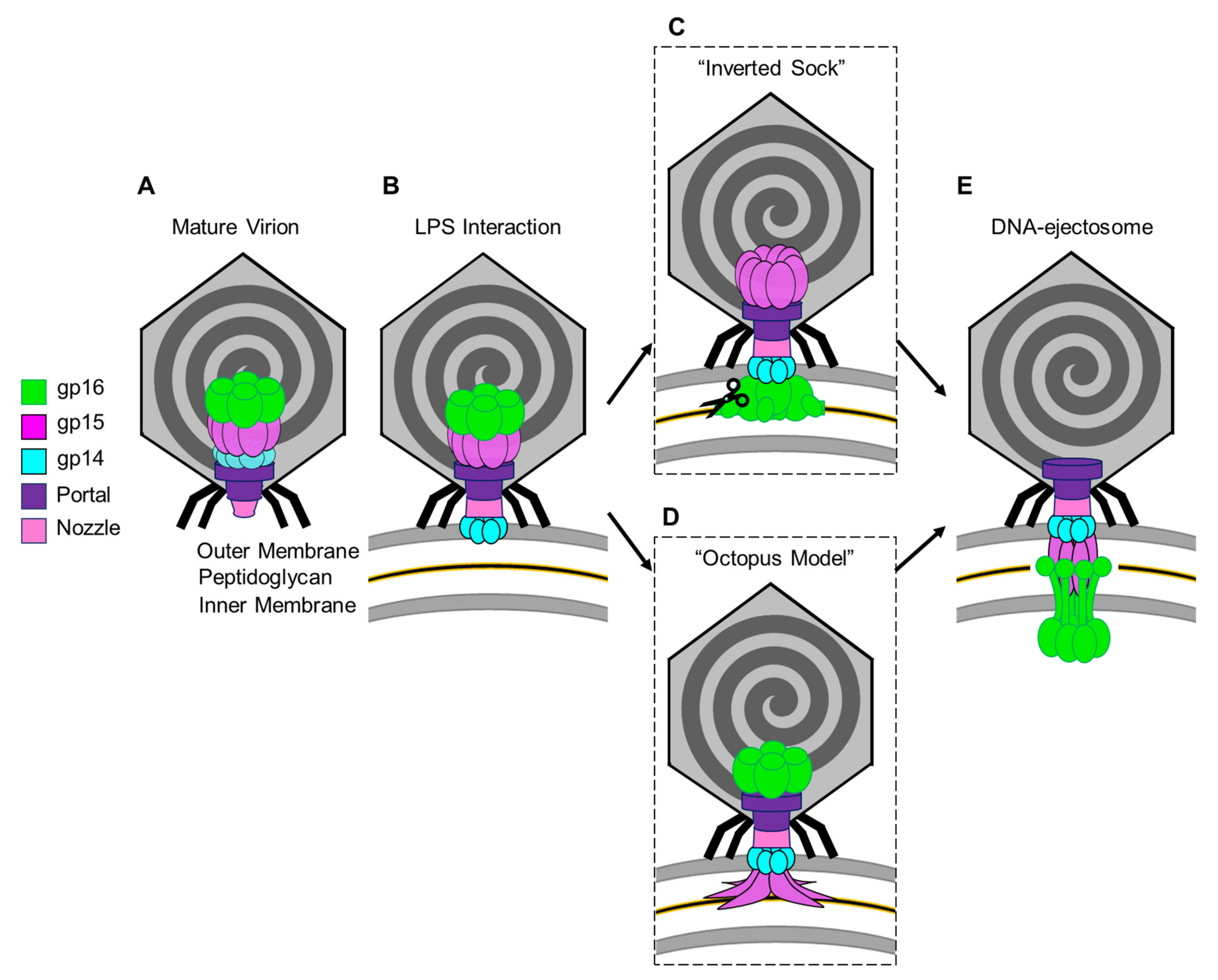
Figure 3. Models for the T7 ejection proteins assembly to form a DNA ejectosome. (A)The mature T7 virion shown with the internal core stack concentrically arranged on the portal protein. (B) Tail fiber interactions with LPS on the host E. coli surface initiate conformational changes in the nozzle and portal protein and eject gp14 to form an outer membrane pore. (C) In the “inverted sock” model, gp16 exits next, in the “inverted sock model”, and cleaves the peptidoglycan layer within the host periplasm, followed by gp15’s exit to form the periplasmic tunnel (PT). (D) Alternatively, in the “octopus model” gp15 exits after gp14, forming a partially stabilized hexamer prior to gp16’s exit and cleavage of the peptidoglycan barrier in the periplasm. (E) Both models end with the formation of the DNA-ejectosome which includes the gp14 outer membrane pore connected to the gp15:gp16 periplamic tunnel which traverses the host inner membrane and projects the gp16-C cytoplasmic hub for viral genome translocation.
Both of these intermediary models end with gp16-C ratcheting in the viral genome in a transcription-independent enzymatic manner until the E. coli RNApol binds to promoters in the viral genome and initiates translocation via the force of transcription in an energy-dependent manner. The leading stretch of the viral genome that the E. coli RNApol transcribes includes the T7 RNApol, which, once assembled, can transcribe the remaining viral genome until completion [19][27]. It was expected that once the viral genome has completely entered the host cytoplasm, the DNA ejectosome may disassemble and remove the IM pore formed by gp16-C in order to maintain cytoplasmic membrane potential.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10030504
References
- Felsenstein, J. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 1996, 266, 418–427.
- Wang, C.; Tu, J.; Liu, J.; Molineux, I.J. Structural dynamics of bacteriophage P22 infection initiation revealed by cryo-electron tomography. Nat. Microbiol. 2019, 4, 1049–1056.
- Wu, W.; Leavitt, J.C.; Cheng, N.; Gilcrease, E.B.; Motwani, T.; Teschke, C.M.; Casjens, S.R.; Steven, A.C. Localization of the Houdinisome (Ejection Proteins) inside the Bacteriophage P22 Virion by Bubblegram Imaging. MBio 2016, 7, e01152-16.
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641.
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321.
- Chang, J.T.; Schmid, M.F.; Haase-Pettingell, C.; Weigele, P.R.; King, J.A.; Chiu, W. Visualizing the structural changes of bacteriophage Epsilon15 and its Salmonella host during infection. J. Mol. Biol. 2010, 402, 731–740.
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A comprehensive, accurate, and fast distance-based phylogeny inference program. Mol. Biol. Evolut. 2015, 32, 2798–2800.
- Swanson, N.A.; Lokareddy, R.K.; Li, F.; Hou, C.-F.D.; Leptihn, S.; Pavlenok, M.; Niederweis, M.; Pumroy, R.A.; Moiseenkova-Bell, V.Y.; Cingolani, G. Cryo-EM structure of the periplasmic tunnel of T7 DNA-ejectosome at 2.7 A resolution. Mol. Cell 2021, 81, 3145–3159.e3147.
- Kemp, P.; Garcia, L.R.; Molineux, I.J. Changes in bacteriophage T7 virion structure at the initiation of infection. Virology 2005, 340, 307–317.
- Chang, C.Y.; Kemp, P.; Molineux, I.J. Gp15 and gp16 cooperate in translocating bacteriophage T7 DNA into the infected cell. Virology 2010, 398, 176–186.
- Leptihn, S.; Gottschalk, J.; Kuhn, A. T7 ejectosome assembly: A story unfolds. Bacteriophage 2016, 6, e1128513.
- Hardies, S.C.; Thomas, J.A.; Black, L.; Weintraub, S.T.; Hwang, C.Y.; Cho, B.C. Identification of structural and morphogenesis genes of Pseudoalteromonas phage phiRIO-1 and placement within the evolutionary history of Podoviridae. Virology 2016, 489, 116–127.
- Dedeo, C.L.; Cingolani, G.; Teschke, C.M. Portal Protein: The Orchestrator of Capsid Assembly for the dsDNA Tailed Bacteriophages and Herpesviruses. Annu. Rev. Virol. 2019, 6, 141–160.
- Perez, G.L.; Huynh, B.; Slater, M.; Maloy, S. Transport of phage P22 DNA across the cytoplasmic membrane. J. Bacteriol. 2009, 191, 135–140.
- Casjens, S.R.; Thuman-Commike, P.A. Evolution of mosaically related tailed bacteriophage genomes seen through the lens of phage P22 virion assembly. Virology 2011, 411, 393–415.
- Zhao, H.; Speir, J.A.; Matsui, T.; Lin, Z.; Liang, L.; Lynn, A.Y.; Varnado, B.; Weiss, T.M.; Tang, L. Structure of a Bacterial Virus DNA-Injection Protein Complex Reveals a Decameric Assembly with a Constricted Molecular Channel. PLoS ONE 2016, 11, e0149337.
- Leiman, P.G.; Battisti, A.J.; Bowman, V.D.; Stummeyer, K.; Muhlenhoff, M.; Gerardy-Schahn, R.; Scholl, D.; Molineux, I.J. The structures of bacteriophages K1E and K1-5 explain processive degradation of polysaccharide capsules and evolution of new host specificities. J. Mol. Biol. 2007, 371, 836–849.
- Moak, M.; Molineux, I.J. Peptidoglycan hydrolytic activities associated with bacteriophage virions. Mol. Microbiol. 2004, 51, 1169–1183.
- Molineux, I.J. No syringes please, ejection of phage T7 DNA from the virion is enzyme driven. Mol. Microbiol. 2001, 40, 1–8.
- Lupo, D.; Leptihn, S.; Nagler, G.; Haase, M.; I, J.M.; Kuhn, A. The T7 ejection nanomachine components gp15-gp16 form a spiral ring complex that binds DNA and a lipid membrane. Virology 2016, 486, 263–271.
- Jiang, W.; Chang, J.; Jakana, J.; Weigele, P.; King, J.; Chiu, W. Structure of epsilon15 bacteriophage reveals genome organization and DNA packaging/injection apparatus. Nature 2006, 439, 612–616.
- Tang, J.; Lander, G.C.; Olia, A.; Li, R.; Casjens, S.; Prevelige, P., Jr.; Cingolani, G.; Baker, T.S.; Johnson, J.E. Peering down the barrel of a bacteriophage portal: The genome packaging and release valve in p22. Structure 2011, 19, 496–502.
- Lokareddy, R.K.; Sankhala, R.S.; Roy, A.; Afonine, P.V.; Motwani, T.; Teschke, C.M.; Parent, K.N.; Cingolani, G. Portal protein functions akin to a DNA-sensor that couples genome-packaging to icosahedral capsid maturation. Nat. Commun. 2017, 8, 14310.
- Chen, W.; Xiao, H.; Wang, L.; Wang, X.; Tan, Z.; Han, Z.; Li, X.; Yang, F.; Liu, Z.; Song, J.; et al. Structural changes in bacteriophage T7 upon receptor-induced genome ejection. Proc. Natl. Acad. Sci. USA 2021, 118, e2102003118.
- Gonzalez-Garcia, V.A.; Pulido-Cid, M.; Garcia-Doval, C.; Bocanegra, R.; van Raaij, M.J.; Martin-Benito, J.; Cuervo, A.; Carrascosa, J.L. Conformational changes leading to T7 DNA delivery upon interaction with the bacterial receptor. J. Biol. Chem. 2015, 290, 10038–10044.
- Perez-Ruiz, M.; Pulido-Cid, M.; Luque-Ortega, J.R.; Valpuesta, J.M.; Cuervo, A.; Carrascosa, J.L. Assisted assembly of bacteriophage T7 core components for genome translocation across the bacterial envelope. Proc. Natl. Acad. Sci. USA 2021, 118, e2026719118.
- Struthers-Schlinke, J.S.; Robins, W.P.; Kemp, P.; Molineux, I.J. The internal head protein Gp16 controls DNA ejection from the bacteriophage T7 virion. J. Mol. Biol. 2000, 301, 35–45.
This entry is offline, you can click here to edit this entry!
