Androgen-deprivation therapy (ADT) is a systemic therapy administered for the management of advanced prostate cancer (PCa). Although ADT may improve survival, long-term use reduces bone mass density (BMD), posing an increased risk of fracture. Considering the long natural history of PCa, it is essential to preserve bone health and quality-of-life in patients on long-term ADT. As an alternative to pharmacological interventions targeted at preserving BMD, current evidence recommends lifestyle modifications, including individualized exercise and nutritional interventions. Exercise interventions include resistance training, aerobic exercise, and weight-bearing impact exercise, and have shown efficacy in preserving BMD. At the same time, it is important to take into account that PCa is a progressive and debilitating disease in which a substantial proportion of patients on long-term ADT are older individuals who harbor axial bone metastases. Smoking cessation and limited alcohol consumption are commonly recommended lifestyle measures in patients receiving ADT. Contemporary guidelines regarding lifestyle modifications vary by country, organization, and expert opinion.
- androgen
- bone diseases
- endocrine
- lifestyle
- prostatic neoplasm
- quality of life
1. Introduction
Prostate cancer (PCa) is the second most commonly diagnosed of all male cancers worldwide and is the fifth leading cause of cancer death in men [1]. Treatment options for PCa patients include surgical procedures, radiation therapy, androgen-deprivation therapy (ADT), and chemotherapy, depending on the stage and course of the disease. Androgen receptor (AR) signaling actively promotes growth, proliferation, and invasiveness of PCa. ADT is most commonly administered as a gonadotrophin-releasing hormone (GnRH) analog, which induces downregulation of the pituitary-gonadal axis and subsequent suppression of the testicular production of testosterone, resulting in chemical castration or the reduction in androgen activity to castration levels. ADT is a standard therapy for patients with aggressive and systematic disease, in which the suppression of androgen activity to castration levels reduces disease-related morbidity and prolongs survival [2].
PCa is an androgen-dependent disease; therefore, ADT is a keystone in the treatment for hormone-sensitive metastatic or advanced disease [3]. Docetaxel chemotherapy and androgen receptor axis-targeted agents, including abiraterone or enzalutamide, have shown clinical efficacy in patients with castration-resistant PCa (CRPC) [4]. Still, ADT is the mainstay treatment for patients with advanced disease or CRPC and should be continuously maintained [4]. Despite the oncological benefits of ADT, long-term administration induces clinical sequelae of reduced quality of life (QoL), sexual dysfunction, and adverse metabolic effects, such as the development of insulin resistance, reduced muscle mass, increased fat mass, and loss of bone mineral density (BMD) [3][4][5][6]. The decline in BMD is a devastating adverse effect of ADT that increases the risks of skeletal-related events (SREs) such as pathological fractures and spinal cord compression. The need for bone surgery or adjunctive treatments for these patients is known to reduce QoL and survival [7].
Loss of BMD in patients on ADT is a commonly overlooked and undertreated condition. Considering the long natural history of PCa, appropriate monitoring and management are essential in preserving bone health and health-related QoL in patients with PCa on long-term ADT. This article will review current guidelines available for the monitoring and management of ADT-induced bone loss in patients with PCa and will explore the effects of current lifestyle modifications and interventions, particularly on dietary supplementation and physical exercise in managing ADT-related alterations to bone health.
2. Pathophysiology Underlying Prostate Cancer Bone Metastasis
The skeletal system is the most common site of PCa metastasis, with 90% of patients with advanced PCa having bone involvement [8]. The most common sites are the vertebral column, pelvic bone, ribs, long bones, and the skull. Bone metastases are associated with SREs, such as pathological fracture, spinal cord compression, and surgical or radiation intervention of the bone. SREs are known to increase morbidity and to negatively impact QoL [9]. The aim of treatment for SREs is focused on delaying the onset of adverse events and maintaining QoL and functional status in patients with bone metastatic PCa [10].
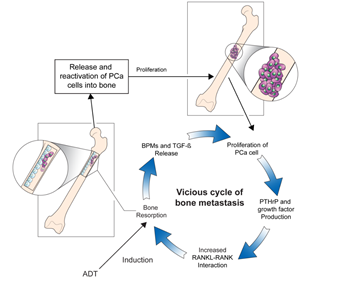
The underlying pathogenesis of bone metastases in patients with PCa remains unclear, and studies are ongoing to elucidate the mechanisms of this entity. High levels of biochemical markers of bone turnover in patients with PCa with bone metastases indicate increased rates of osteoblast and osteoclast activity [11]. Moreover, researchers have hypothesized that interactions between the bone microenvironment and cancer cells may induce a vicious cycle of bone destruction and metastasis of the tumor [12]. Circulating tumor cells secrete interleukins, a gradient of chemokines, and parathyroid hormone-related protein (PTH-rP), which initiates osteolysis and results in the release of cytokines and factors by the bone marrow. The receptor activator of nuclear factor-ĸβ ligand (RANKL) is a mediator of bone remodeling and binds to its receptor on the surface of osteoclast progenitors, which leads to bone resorption. Tumor cells increase RANKL expression on osteoblasts by secreting PTH-rP, which leads to the proliferation of osteoclasts and increased bone resorption. In turn, osteoclast precursors, such as bone morphogenetic proteins, fibroblast growth factor, platelet-derived growth factor, and transforming growth factor-β, are activated. This stimulates tumor cell proliferation and the production of resorptive factors, completing the vicious cycle of tumor metastasis and osteolysis (Figure 1) [13]. Further studies focusing on the underlying pathophysiology directing the progression of bone metastases in patients with PCa would be crucial to broadening the horizon of novel therapeutic strategies [14].
Figure 1. The vicious cycle of bone metastasis. Bone resorption releases and reactivates PCa cells into the bone, leading to metastatic outgrowth in the bone microenvironment. Production of factors by PCa cells that increase bone resorption through enhanced interaction between RANKL expressing osteoblasts and RANK expressing osteoclasts mediates the vicious cycle of PCa bone metastasis. Production of PTHrP and growth factors increases the interaction between RANKL-expressing osteoblasts and RANK-expressing osteoclasts, which further increases bone resorption. The release of BMPs and TGF-β by bone resorption further aggravates the vicious cycle. ADT: androgen-deprivation therapy, BMPs: bone morphogenetic proteins, PCa: prostate cancer, PTHrP: parathyroid hormone-related protein, RANK: receptor activator of nuclear factor-ĸβ, RANKL: receptor activator of nuclear factor-ĸβ ligand, TGF-β: transforming growth factor-β.
3. Pathophysiology of Cancer Treatment-Induced Bone Loss
The progression of PCa is an androgen-dependent process. After the administration of ADT, sex steroid levels rapidly decrease and reach a nadir within four weeks of therapy [15]. The interactions between bone homeostasis and sex steroids have been widely explored [16][17][18][19]. In the skeletal system, androgens directly stimulate growth plate chondrocytes and influence the longitudinal growth of bone [20]. AR signaling in osteoblasts promotes differentiation and increases the protective effects of androgens on trabecular bone mass, which in turn decreases bone resorption and osteoclast numbers [21]. The role of estrogens in bone is to protect against endocortical resorption, which is regulated by estrogen receptors (ERα, ERβ) that are expressed by mesenchymal or stromal cells [22]. Estrogens play an essential role in osteoblast proliferation along with the inhibition of osteoclast precursors and in the regulation of osteoclast apoptosis[22][23]. Accumulating evidence supports the hypothesis that compared to testosterone, estrogen plays a more influential role in the regulation of bone metabolism in males [24][25][26].
Following the administration of ADT, serum levels of testosterone and estradiol show a substantial and rapid decrease, which leads to disruptions of skeletal homeostasis and bone rigidity. A prospective trial conducted by Greenspan et al. evaluated the rate of bone loss after ADT administration in patients with PCa and observed that the reduction in BMD was most rapid during the first year of ADT initiation, suggesting that preventive measures should be considered during this early period [15]. The duration of ADT and the harmful side effects on bone health show a direct relationship. In a separate cross-sectional study reported by Kiratli et al., a constant reduction in BMD was observed in patients with PCa who received long-term ADT. This effect was more prominent in patients who underwent continuous ADT and surgical castration in comparison to those who received intermittent ADT [27].
Bone loss induced by glucocorticoids is caused by enhanced rates of osteoblast and osteocyte apoptosis, impaired osteoblast differentiation, and prolonged osteoclast activity [28]. Glucocorticoids play a distinctive role in the management of CRPC, where they are used in combination with chemotherapy or administered as low dosage monotherapy [29][30]. Future investigations are warranted to explore management strategies to reduce the adverse effects of glucocorticoids on skeletal health when co-administered with ADT or adjuvant chemotherapy.
Androgen receptor axis targeted agents, including enzalutamide and abiraterone acetate in combination with prednisone, have gained FDA approval for their survival benefit in patients with advanced disease. In addition to their survival benefits, pooled analysis investigating the impact of both agents on skeletal endpoints has demonstrated an advantage in terms of SREs [31]. It is unclear whether this effect is secondary to the systemic control of bone metastasis or a direct effect on the bone microenvironment. In need of reducing the adverse metabolic effects of ADT, several studies have investigated the effect of these agents on bone metabolism. A single-arm, phase II trial investigated the potential of enzalutamide as a monotherapy for patients with hormone-naïve PCa eligible for ADT [32]. Bone turnover and metabolic outcomes were included in the exploratory outcomes, which indicated a stable BMD, with only small changes comparable to those achieved with bicalutamide and in contrast to those for leuprolide. Increased levels of estrogen and bone turnover markers of bone resorption with enzalutamide monotherapy were suggested as the pathophysiological mechanism of BMD maintenance [33]. Considering that enzalutamide is administered without the need for glucocorticoids, future trials would be warranted to investigate the long-term efficacy and safety of enzalutamide as monotherapy in terms of survival and cancer treatment-induced bone loss (CTIBL). For abiraterone acetate, the results from an in vitro study suggest a direct bone anabolic and an anti-resorptive effect [34]. Furthermore, abiraterone acetate was found to exhibit an inhibitory effect on human primary osteoclast function and to promote osteoblast differentiation and bone matrix deposition in patients with mCRPC [31]. Future studies are warranted to investigate how abiraterone acetate and enzalutamide may be utilized to exert additional positive effects on CTIBL due to ADT.
The increased adaptive exposure of ARs results in an incremental resistance to ADT. Evidence has demonstrated that this increase produces a therapeutic vulnerability, which leads to cellular apoptosis induced by supraphysiologic levels of androgens [35][36]. Bipolar androgen therapy has been proposed as an alternative therapeutic approach, in which rapid hormonal cycling between supraphysiologic and castrate levels of testosterone is performed to disrupt adaptive regulation of AR levels [37]. The BATMAN study was a phase II trial that investigated the feasibility of bipolar androgen therapy in terms of prostate-specific antigen response and metabolic effects in patients with hormone-sensitive PCa [38]. BMD was measured using dual-energy X-ray absorptiometry (DXA) at baseline and 6 and 15 months. As expected, there was no decline in BMD throughout the study period. Of note, the relatively small sample size and a short follow-up period of the study may have limited the ability to detect changes in bone metabolism.
Apalutamide is another competitive inhibitor of the AR. SPARTAN was a phase III trial that demonstrated prolonged metastatic-free survival and time to symptomatic progression in patients with non-metastatic CPRC [39]. Despite the survival benefit, the results indicate that treatment with apalutamide increased the risk of non-pathological bone fractures. The exact pathophysiology underlying non-pathological fractures is unclear; however, it may be presumable that more potent inhibition of testosterone activity may have accelerated bone turnover and subsequent fragility of the bone matrix.
This entry is adapted from the peer-reviewed paper 10.3390/cancers12061529
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424, doi:10.3322/caac.21492.
- Koo, K.C.; Park, S.U.; Kim, K.H.; Rha, K.H.; Hong, S.J.; Yang, S.C.; Chung, B.H. Predictors of survival in prostate cancer patients with bone metastasis and extremely high prostate-specific antigen levels. Prostate Int. 2015, 3, 10–15, doi:10.1016/j.prnil.2015.02.006.
- Singh, J.; Trabulsi, E.J.; Gomella, L.G. The Quality-of-Life Impact of Prostate Cancer Treatments. Curr. Urol. Rep. 2010, 11, 139–146, doi:10.1007/s11934-010-0103-y.
- Bourke, L.; Boorjian, S.A.; Briganti, A.; Klotz, L.; Mucci, L.; Resnick, M.J.; Rosario, D.J.; Skolarus, T.A.; Penson, D. Survivorship and Improving Quality of Life in Men with Prostate Cancer. Eur. Urol. 2015, 68, 374–383, doi:10.1016/j.eururo.2015.04.023.
- Kim, D.K.; Lee, J.Y.; Kim, K.J.; Hong, N.; Kim, J.W.; Hah, Y.S.; Koo, K.C.; Kim, J.H.; Cho, K.S. Effect of Androgen-Deprivation Therapy on Bone Mineral Density in Patients with Prostate Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 113, doi:10.3390/jcm8010113.
- Nguyen, P.L.; Alibhai, S.M.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.; Rosario, D.J.; Tombal, B.; Smith, M.R. Adverse Effects of Androgen Deprivation Therapy and Strategies to Mitigate Them. Eur. Urol. 2015, 67, 825–836, doi:10.1016/j.eururo.2014.07.010.
- So, A.; Chin, J.; Fleshner, N.; Saad, F. Management of skeletal-related events in patients with advanced prostate cancer and bone metastases: Incorporating new agents into clinical practice. Can. Urol. Assoc. J. 2012, 6, 465–470.
- 8. Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. New Engl. J. Med. 2004, 351, 1502–1512, doi:10.1056/nejmoa040720.
- Saad, F.; Ivanescu, C.; Phung, D.; Loriot, Y.; Abhyankar, S.; Beer, T.M.; Tombal, B.; Holmstrom, S. Skeletal-related events significantly impact health-related quality of life in metastatic castration-resistant prostate cancer: Data from PREVAIL and AFFIRM trials. Prostate Cancer Prostatic Dis. 2017, 20, 110–116, doi:10.1038/pcan.2016.62.
- Vignani, F.; Bertaglia, V.; Buttigliero, C.; Tucci, M.; Scagliotti, G.V.; Di Maio, M. Skeletal metastases and impact of anticancer and bone-targeted agents in patients with castration-resistant prostate cancer. Cancer Treat. Rev. 2016, 44, 61–73, doi:10.1016/j.ctrv.2016.02.002.
- Cook, R.J.; Coleman, R.; Brown, J.E.; Lipton, A.; Major, P.; Hei, Y.J.; Saad, F.; Smith, M.R. Markers of Bone Metabolism and Survival in Men with Hormone-Refractory Metastatic Prostate Cancer. Clin. Cancer Res. 2006, 12, 3361–3367, doi:10.1158/1078-0432.ccr-06-0269.
- Roodman, G.D. Mechanisms of Bone Metastasis. N. Engl. J. Med. 2004, 350, 1655–1664, doi:10.1056/nejmra030831.
- Sottnik, J.L.; Dai, J.; Zhang, H.; Campbell, B.; Keller, E.T. Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. 2015, 75, 2151–2158, doi:10.1158/0008-5472.CAN-14-2493.
- Park, S.H.; Keller, E.T.; Shiozawa, Y. Bone Marrow Microenvironment as a Regulator and Therapeutic Target for Prostate Cancer Bone Metastasis. Calcif. Tissue Int. 2017, 102, 152–162, doi:10.1007/s00223-017-0350-8.
- Greenspan, S.L.; Coates, P.; Sereika, S.M.; Nelson, J.B.; Trump, N.L.; Resnick, N.M. Bone Loss after Initiation of Androgen Deprivation Therapy in Patients with Prostate Cancer. J. Clin. Endocrinol. Metab. 2005, 90, 6410–6417, doi:10.1210/jc.2005-0183.
- Almeida, M.; Laurent, M.R.; Dubois1 † V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187, doi:10.1152/physrev.00033.2015.
- Mohamad, N.V.; Soelaiman, I.-N.; Chin, K.-Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324, doi:10.2147/CIA.S115472.
- Vanderschueren, D.; Laurent, M.R.; Claessens, F.; Gielen, E.; Lagerquist, M.; Vandenput, L.; Börjesson, A.E.; Ohlsson, C. Sex steroid actions in male bone. Endocr. Rev. 2014, 35, 906–960, doi:10.1210/er.2014-1024.
- Wu, J.; Henning, P.; Sjögren, K.; Koskela, A.; Tuukkanen, J.; Movérare-Skrtic, S.; Ohlsson, C. The androgen receptor is required for maintenance of bone mass in adult male mice. Mol. Cell. Endocrinol. 2019, 479, 159–169, doi:10.1016/j.mce.2018.10.008.
- Clarke, B.L.; Khosla, S. Androgens and bone. Steroids 2008, 74, 296–305, doi:10.1016/j.steroids.2008.10.003.
- Kasperk, C.; Wakley, G.K.; Huempfner-Hierl, H.; Ziegler, R. Gonadal and Adrenal Androgens Are Potent Regulators of Human Bone Cell Metabolism In Vitro. J. Bone Miner. Res. 1997, 12, 464–471, doi:10.1359/jbmr.1997.12.3.464.
- Khosla, S. New Insights Into Androgen and Estrogen Receptor Regulation of the Male Skeleton. J. Bone Miner. Res. 2015, 30, 1134–1137, doi:10.1002/jbmr.2529.
- Todenhöfer, T.; Stenzl, A.; Hofbauer, L.C.; Rachner, T.D. Targeting Bone Metabolism in Patients with Advanced Prostate Cancer: Current Options and Controversies. Int. J. Endocrinol. 2015, 2015, 1–9, doi:10.1155/2015/838202.
- Argoud, T.; Boutroy, S.; Claustrat, B.; Chapurlat, R.; Szulc, P. Association Between Sex Steroid Levels and Bone Microarchitecture in Men: The STRAMBO Study. J. Clin. Endocrinol. Metab. 2014, 99, 1400–1410, doi:10.1210/jc.2013-3233.
- Drake, M.T.; Khosla, S. Male osteoporosis. Endocrinol. Metab. Clin. North Am. 2012, 41, 629–641, doi:10.1016/j.ecl.2012.05.001.
- Piot, A.; Chapurlat, R.; Claustrat, B.; Szulc, P. Relationship Between Sex Steroids and Deterioration of Bone Microarchitecture in Older Men: The Prospective STRAMBO Study. J. Bone Miner. Res. 2019, 34, 1562–1573, doi:10.1002/jbmr.3746.
- Kiratli, B.; Srinivas, S.; Perkash, I.; Terris, M.K. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology 2001, 57, 127–132, doi:10.1016/s0090-4295(00)00895-5.
- Uyl, D.D.; Bultink, I.; Lems, W. Advances in Glucocorticoid-Induced Osteoporosis. Curr. Rheumatol. Rep. 2011, 13, 233–240, doi:10.1007/s11926-011-0173-y.
- Auchus, R.J.; Yu, M.K.; Nguyen, S.; Mundle, S.D. Use of Prednisone With Abiraterone Acetate in Metastatic Castration‐Resistant Prostate Cancer. Oncology 2014, 19, 1231–1240, doi:10.1634/theoncologist.2014-0167.
- Dorff, T.; Crawford, E.D. Management and challenges of corticosteroid therapy in men with metastatic castrate-resistant prostate cancer. Ann. Oncol. 2013, 24, 31–38, doi:10.1093/annonc/mds216.
- Rizzo, S.; Galvano, A.; Pantano, F.; Iuliani, M.; Vincenzi, B.; Passiglia, F.; Spoto, S.; Tonini, G.; Bazan, V.; Russo, A.; et al. The effects of enzalutamide and abiraterone on skeletal related events and bone radiological progression free survival in castration resistant prostate cancer patients: An indirect comparison of randomized controlled trials. Crit. Rev. Oncol. 2017, 120, 227–233, doi:10.1016/j.critrevonc.2017.09.008.
- Tombal, B.; Borre, M.; Rathenborg, P.; Werbrouck, P.; Van Poppel, H.; Heidenreich, A.; Iversen, P.; Braeckman, J.; Heracek, J.; Baskin-Bey, E.; et al. Enzalutamide monotherapy in hormone-naive prostate cancer: Primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol. 2014, 15, 592–600, doi:10.1016/s1470-2045(14)70129-9.
- Smith, M.R.; Goode, M.; Zietman, A.L.; McGovern, F.J.; Lee, H.; Finkelstein, J.S. Bicalutamide Monotherapy Versus Leuprolide Monotherapy for Prostate Cancer: Effects on Bone Mineral Density and Body Composition. J. Clin. Oncol. 2004, 22, 2546–2553, doi:10.1200/jco.2004.01.174.
- Iuliani, M.; Pantano, F.; Buttigliero, C.; Fioramonti, M.; Bertaglia, V.; Vincenzi, B.; Zoccoli, A.; Ribelli, G.; Tucci, M.; Vignani, F.; et al. Biological and clinical effects of abiraterone on anti-resorptive and anabolic activity in bone microenvironment. Oncotarget 2015, 6, 12520–12528, doi:10.18632/oncotarget.3724.
- Kokontis, J.M.; Hay, N.; Liao, S. Progression of LNCaP Prostate Tumor Cells during Androgen Deprivation: Hormone-Independent Growth, Repression of Proliferation by Androgen, and Role for p27Kip1 in Androgen-Induced Cell Cycle Arrest. Mol. Endocrinol. 1998, 12, 941–953, doi:10.1210/mend.12.7.0136.
- Schweizer, M.T.; Antonarakis, E.S.; Wang, H.; Ajiboye, A.S.; Spitz, A.; Cao, H.; Luo, J.; Haffner, M.C.; Yegnasubramanian, S.; Carducci, M.A.; et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: Results from a pilot clinical study. Sci. Transl. Med. 2015, 7, 269ra2, doi:10.1126/scitranslmed.3010563.
- Denmeade, S.R.; Isaacs, J.T. Bipolar androgen therapy: The rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant prostate cancer. Prostate 2010, 70, 1600–1607, doi:10.1002/pros.21196.
- Schweizer, M.T.; Wang, H.; Luber, B.; Nadal, R.; Spitz, A.; Rosen, D.M.; Cao, H.; Antonarakis, E.S.; Eisenberger, M.A.; Carducci, M.A.; et al. Bipolar Androgen Therapy for Men With Androgen Ablation Naïve Prostate Cancer: Results From the Phase II BATMAN Study. Prostate 2016, 76, 1218–1226, doi:10.1002/pros.23209.
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418, doi:10.1056/nejmoa1715546.