Hypoxic-ischemic encephalopathy (HIE) following perinatal asphyxia is a major cause of neurological sequelae in term and near-term infants. Despite therapeutic hypothermia, a significant number of infants still have adverse outcomes. Neuroimaging is the standard of care in infants with HIE to determine the nature and timing of the injury, guide further treatment, and predict neurodevelopmental outcomes. Cranial ultrasonography is helpful to assess the brain before initiation of therapeutic hypothermia to look for abnormalities suggestive of antenatal onset of injury or HIE mimics. However, magnetic resonance imaging (MRI) which includes diffusion-weighted imaging has become the gold standard to assess brain injury in newborns with HIE, and has an excellent prognostic utility. Magnetic resonance spectroscopy provides complementary metabolic information and has also been shown to be a reliable prognostic biomarker. Advanced imaging modalities, such as diffusion tensor imaging and arterial spin labeling, are increasingly being used to gain further information about the etiology and prognosis of brain injury in infants with HIE due to perinatal asphyxia.
1. Brain Injury Patterns in HIE
The extent and location of brain injury in newborns with hypoxic-ischemic encephalopathy (HIE) due to perinatal asphyxia is dependent on the timing, severity, and duration of the hypoxic-ischemic event, in addition to the brain maturation at the time the insult occurs [
20]. Three main patterns of injury can be distinguished in newborns with HIE:
1.1. Basal Ganglia and Thalami (BGT) Predominant Pattern of Injury
Several conditions can lead to an abrupt impairment of placental perfusion or oxygen delivery in the umbilical cord, resulting in acute profound asphyxia. These conditions include among others placental abruption, umbilical cord prolapse, shoulder dystocia and uterine rupture. As there is insufficient time to redirect blood flow through autoregulation, these sentinel events will especially affect the brain areas with the highest metabolic demand. These include the basal ganglia and thalami (BGT), mainly the ventrolateral thalami and posterior putamina, and the perirolandic cortex (
Figure 1) [
21]. This pattern of injury is associated with impaired motor neurodevelopment, including cerebral palsy (CP) [
22,
23]. A strong association between the severity of BGT abnormalities and the severity of motor impairment has been demonstrated, with a predictive accuracy of severe BGT lesions being 0.89 (95% CI 0.83–0.96) for severe motor impairment [
22].
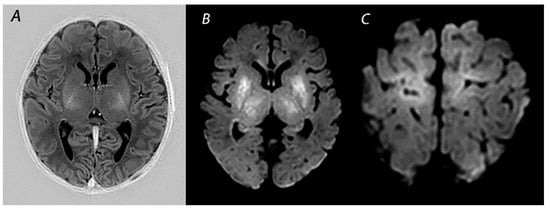
Figure 1. MRI performed on day 5 in an infant who received therapeutic hypothermia for HIE. Although the signal intensity of the posterior limb of the internal capsule appears normal on T1-weighted imaging (A), DWI shows extensive diffusion restriction in the basal ganglia and thalami (B), and the perirolandic cortex (C). The infant died after redirection of care.
1.2. White Matter/Watershed (WM/WS) Predominant Pattern of Injury
Mild to moderate asphyxia, also known as partial prolonged asphyxia, allows time for cerebral autoregulation to redirect blood flow to the brain areas with the highest metabolic demand, at the cost of the watershed areas of the anterior, middle, and posterior cerebral arteries [
20]. This results in a watershed predominant pattern of injury (
Figure 2). As injury follows a parasagittal distribution along these vascular border zones, this pattern is also known as the “parasagittal pattern of injury” [
24]. In the more severely affected infants, injury can also be seen in the parasagittal cortex and the subcortical white matter. Physiological and neurological manifestations in infants with WM/WS predominant pattern of injury are often not severe and only present for a short time after birth, in contrast to infants with BGT pattern of injury who in general have lower Apgar scores, need more intensive resuscitation at birth, and present with more severe encephalopathy [
25]. Nevertheless, clinical and neurological abnormalities may progress over time, highlighting the importance of careful observation of infants with perinatal asphyxia [
26]. Severe motor impairment is rare in infants with mild to moderate WM/WS pattern of injury [
25,
27]. At early follow-up around 12–18 months after birth, they are often considered to have a normal outcome [
28]. However, these infants are likely to grow into their deficits and develop cognitive problems over the years. Long-term follow-up to detect disabilities early in life and ensure an early start of interventions is therefore important [
25,
27,
29,
30].
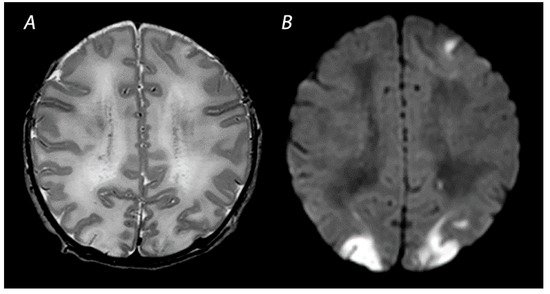
Figure 2. MRI performed on day 4 in an infant with a watershed predominant pattern of injury, with T2-weighted imaging showing loss of distinction between the gray and white matter at the cortex of the occipital lobes and the left frontal lobe (A) and DWI (B) showing diffusion restriction in these areas.
Some infants with HIE show abnormalities restricted to the periventricular white matter, including punctate white matter lesions (PWMLs), small focal patches of increased signal intensity on T1-weighted imaging, and decreased intensity on T2-weighted imaging [
31,
32]. Hayman et al. studied 44 (near-)term infants with PWMLs, of whom 45% were associated with perinatal asphyxia [
31]. None of these infants had evidence of BGT injury or another more extensive pattern of injury. The majority of infants in this study had normal early outcomes or mild neurologic deficits. Most of those who experienced severe neurological sequelae had an underlying genetic disorder.
1.3. Near Total Injury
Near total injury, which is also referred to as “global injury”, is characterized by diffuse injury in both the BGT and white matter. This pattern is seen in newborns affected by very severe and prolonged asphyxia. It is less often reported compared to BGT or WM/WS patterns of injury, as infants with near total injury may be too ill to be scanned after birth and pass away before MRI can be performed [
25] (
Figure 3). The cerebellum may look relatively normal on DWI in contrast to a completely white cerebrum, which is why this pattern is also being referred to as the “white cerebrum” [
33]. Nevertheless, it has been demonstrated that cerebellar abnormalities may be underestimated on DWI, and that only in cases of very severe cytotoxic edema abnormalities are found [
34]. There is growing evidence for a superior role of advanced MRI modalities, such as quantitative diffusion tensor imaging (DTI) analysis, to adequately detect cerebellar injury [
35].
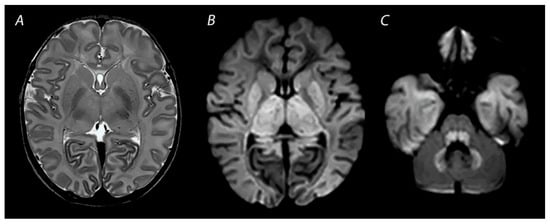
Figure 3. MRI performed on day 3 after birth, showing near total injury with extensive injury in the white matter, BGT, brainstem and cerebellum. Abnormalities are often more subtle on T2 weighted imaging in the first days after the hypoxic-ischemic insult (A) and are best seen using DWI on early MRI (B,C). Except for the dentate nuclei, the signal in the cerebellum is not increased and therefore the term ‘the white brain’ was coined. The infant died after redirection of care.
1.4. Other Injury Associated with HIE
Vascular abnormalities, including perinatal arterial ischemic stroke, perinatal hemorrhagic stroke, and sinovenous thrombosis, have also been demonstrated in infants with encephalopathy due to perinatal asphyxia [
36,
37]. Previous studies have demonstrated perinatal stroke in 4–5% of newborns with HIE [
37,
38] (
Figure 4). Whether there is a causal relationship between perinatal asphyxia and neonatal stroke is controversial. Asphyxia has been proposed to be involved in the development of neonatal stroke in experimental and clinical studies [
39,
40]. Findings from a study by Harbert and colleagues have suggested that therapeutic hypothermia could potentially reduce seizures after perinatal stroke [
41]. However, the exact etiology of this disease remains to be further elucidated.
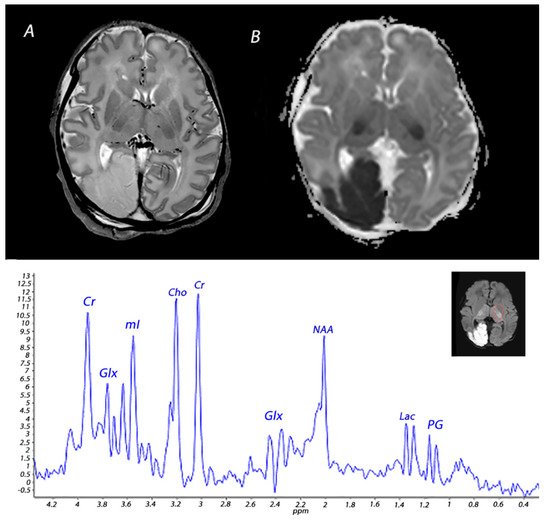
Figure 4. MRI performed on day 3 in an infant who received therapeutic hypothermia for severe HIE. MRI showed posterior cerebral artery stroke, presenting as increased signal intensity and loss of cortical gray and white matter differentiation on T2 weighted imaging (A) and a low signal intensity on ADC mapping (B). Injury was also noted in the BGT. 1H-MRS performed in the BGT shows a lactate peak, presenting as a doublet peak at 1.33 ppm.
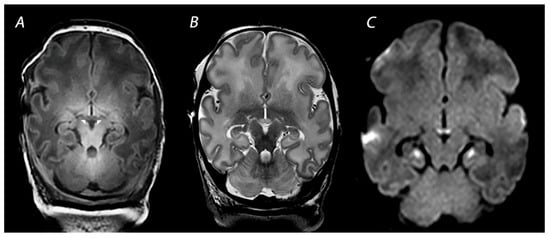
Some infants with HIE show brain injury in the limbic system, particularly in the hippocampal region, which can lead to long-term memory impairment at school age [
42,
43]. Additionally, a recent study has demonstrated that the mammillary bodies (MB), which are also part of the limbic circuit and play an important role in episodic memory, are often injured in infants with HIE [
44] (
Figure 5). Molavi and colleagues showed that 13% of full-term infants with HIE had abnormal signal intensity on T2-weighted imaging. In all but one of the infants with follow-up MRI, these initial signal abnormalities resulted in MB atrophy [
44]. In another study across multiple centers, it was demonstrated that approximately 40% of infants with HIE who had received hypothermia had signal abnormalities in the MBs [
45]. Annink et al. reported that MB atrophy was significantly associated with lower intelligence quotients, verbal long-term memory scores, and visual-spatial long-term memory scores at 10 years of age [
46].
Figure 5. MRI performed on day 10 showing injury of the mammillary bodies, presenting as decreased signal intensity on T1 weighted imaging as indicated by the white arrows on (A), swelling and increased signal intensity on T2-weighted imaging (B), and diffusion restriction on diffusion-weighted imaging (C). Diffusion restriction is also noted in the hippocampi, which is often seen in combination with mammillary body injury.
2. Magnetic Resonance Imaging in Infants with HIE
2.1. Conventional MRI
MRI has been performed in infants with HIE since the late 1980s, and initially included T1- and T2-weighted imaging [
47]. The introduction of DWI has greatly improved the assessment of brain lesions in newborns with asphyxia. T1-weighted imaging aids in the assessment of myelination, the depiction of ischemia, and the detection of subacute hemorrhage in regions with important prognostic implications, such as the BGT and the brainstem [
48]. T2-weighted sequences are useful to identify white matter abnormalities and to assess the gray-white matter differentiation at the cortex [
48]. Abnormalities in this delineation can reveal cortical injury, also referred to as “loss of the cortical ribbon” (
Figure 2). During the first days after birth, the abnormalities on T1- and T2-weighted imaging are often subtle, but they become gradually more apparent by the second half of the first week [
49]. It has been demonstrated that the absence of normal high-signal intensity of the posterior limb of the internal capsule (PLIC) on T1- and T2-weighted imaging is highly predictive of severe adverse motor outcomes [
22,
50]. However, these results derived from studies performing MRI in both the first and second week after birth, and one should be aware that signal abnormalities of the PLIC may take up to 4 days to become visible when only T1- and T2-weighted imaging are being used (
Figure 1) [
22].
2.2. Diffusion Weighted Imaging
DWI is an imaging modality based upon the measurement of the random motion of water molecules within a voxel of brain tissue, providing information on the functional state of the brain tissue. The apparent diffusion coefficient (ADC) is an indicator of the magnitude of movement of water protons within the tissue. As the ADC indicates the overall diffusion restriction, low ADC values will cause a high-signal intensity on isotropic DWI [
51]. A variety of studies have demonstrated restricted water diffusion due to hypoxic-ischemic injury, which can be visible at an early stage when conventional T1 and T2 sequences are still inconspicuous [
52,
53]. In general, abnormalities seen on DWI peak at 3–5 days after the insult and subsequently normalize at approximately 11–12 days for infants treated with therapeutic hypothermia and 6–8 days in non-cooled infants. One must keep in mind that DWI obtained within the first day after birth and near the time of pseudo-normalization may lead to an underestimation of the extent of brain injury [
54].
2.3. Susceptibility Weighted Imaging
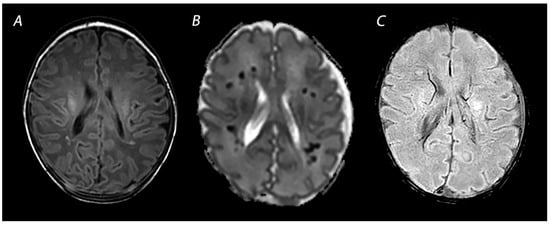
Susceptibility weighted imaging (SWI) is a three-dimensional MRI modality with high spatial resolution and flow compensation that accentuates the magnetic properties of blood, iron, blood products, and calcifications [
55]. It is exquisitely useful to detect vascular abnormalities, such as hemorrhages and cerebral vascular malformations (
Figure 6). As this sequence is very sensitive to the detection of blood oxygenation changes within the veins, due to the increased magnetic susceptibility of deoxygenated blood, it can also play an important role in the early detection of HIE and prognostication [
56,
57]. The compensatory increase in the oxygen extraction fraction following hypoxia-ischemia results in an increased level of deoxygenated hemoglobin in the cerebral vessels draining the injured areas. This may be depicted in SWI as prominent hypo-intense veins. It has been demonstrated that SWI may reveal vascular brain abnormalities even before cytotoxic edema is visible on DWI [
58].
Figure 6. MRI performed on day 7 showing punctate white matter lesions. While these lesions are subtle on T1 weighted imaging (A), the abnormalities are much clearer and more numerous on the ADC map (B). Susceptibility weighted imaging can be used to rule out hemorrhagic abnormalities (C).
3. Advanced Imaging Modalities
3.1. Diffusion Tensor Imaging
In contrast to DWI, DTI also accounts for the effects of the diffusion directionality of water. This is expressed as the functional anisotropy (FA). DTI is a powerful tool to assess microstructural abnormalities of the brain. However, while abnormalities on DWI can be interpreted visually, the acquisition and analysis of DTI data are significantly more complex and, in general, require a reliable quantitative analysis method to draw meaningful conclusions [
59]. One of these methods is the region of interest analysis, in which diffusion measures are obtained from a specific brain region, defined by manual delineation or automated parcellation or segmentation. In a systematic review on DTI, Dibble et al. identified three key regions of interest showing altered diffusion in infants with HIE: the PLIC, and the genu and splenium of the corpus callosum [
14]. Lower FA values in these areas were associated with worse neurodevelopmental outcomes. Another method for quantitative analysis is tract-based spatial statistics (TBSS). This is an automated objective observer-independent whole-brain approach that analyzes aligned FA images from multi-subject diffusion data, allowing powerful comparisons of DTI without requiring a subjective prespecification of regions of interest [
59]. Using TBSS, Porter et al. demonstrated widespread microstructural abnormalities in white matter tracts in term infants with HIE, which were reduced after therapeutic hypothermia, showing that TBSS is suitable to compare white matter tissue integrity among infants and a promising biomarker for early phase trials of new neuroprotective strategies [
60]. In another study including 43 infants with HIE, Tusor and colleagues demonstrated that DTI analyzed by TBSS could predict 2-year outcomes of these infants, with those having an adverse outcome showing significantly lower FA values in the corpus callosum, the centrum semiovale, anterior and posterior limb of the internal capsule, cerebral peduncles, external capsule, optic radiation, fornix, cingulum and inferior longitudinal fasciculus [
18].
3.2. Arterial Spin Labeling
Arterial spin labelin (ASL) is a noninvasive technique to measure brain perfusion, by using magnetically labeled water protons as a tracer [
61]. Following the acute phase of injury after the hypoxic-ischemic event, impaired autoregulation often results in cerebral hyperperfusion, which can lead to delayed brain injury and correlates with an adverse neurodevelopmental outcome [
62].
Perfusion abnormalities visualized with ASL have been shown to aid in early diagnosis and prognostication of infants with HIE [
16,
63,
64]. Wintermark et al. assessed brain perfusion using ASL in the first week after birth in 18 infants with perinatal asphyxia, of whom 11 were treated with hypothermia and 4 were control patients, and demonstrated that abnormal cerebral blood flow values can precede injury seen on later MRI [
63]. Cooled infants who developed evidence of hypoxic-ischemic brain injury on MRI showed more markedly decreased perfusion on the first day after birth in the brain areas subsequently exhibiting injury, in comparison to the control group and the cooled infants who did not develop brain injury. On the second day after birth, all but one of these infants demonstrated hyperperfusion in these injured brain areas, suggesting that an initial hypoperfusion phase followed by a hyperperfusion phase in specific brain regions correlates with the development of injury in those regions. Newborns with perinatal asphyxia who did not receive hypothermia therapy and developed brain injury showed hyperperfusion on days 1–6 after birth in the brain areas showing injury on MRI. Another study evaluated the predictive value of ASL in 28 infants with HIE and reported that perfusion in the BGT was higher in those with an adverse outcome at 9–18 months of age than in the neonates with a favorable outcome [
16]. When comparing the predictive value of ASL with other MRI predictive markers, the combination of ASL with Lactate/N-acetylaspartate (NAA) measured by
1H-MRS, was demonstrated to be the best outcome predictor. ASL seems most relevant when performed in the first days after the hypoxic-ischemic insult. Based on a study on newborns with HIE who underwent MRI around 4 days and 11 days after birth, Proisy et al. demonstrated that cerebral blood flow levels measured in the BGT around day 4 were significantly higher in infants with ischemic lesions on MRI compared to infants with normal MRI [
65]. No significant difference in cerebral blood flow levels measured in the BGT around 11 days after birth was demonstrated between these groups. However, this could also be due to the small sample size of this study.
4. Magnetic Resonance Spectroscopy
Nuclear magnetic resonance spectroscopy of protons, referred to as
1H-MRS, has been used since the early 1990s to obtain metabolic information about brain metabolism and detect changes in neurochemistry caused by asphyxia [
66]. The magnetic field that a particular proton experiences is influenced by its chemical environment. Differently sited protons will therefore resonate at different frequencies. This so-called chemical shift is used in
1H-MRS to discern different metabolites. There is compelling evidence for the excellent prognostic value of
1H-MRS in newborns with HIE [
13,
14]. NAA and lactate measured in the deep gray matter have been demonstrated to be the most useful biomarkers in the assessment of metabolic changes associated with HIE [
15]. The predictive value for an adverse outcome is excellent, and not affected by therapeutic hypothermia [
13,
15]. These metabolites have a long T2 relaxation time and are therefore best detected using a long (>130 ms) echo-time, which improves the signal-to-noise ratio of these metabolites due to the attenuation of signals from metabolites with a shorter time of relaxation.
NAA is found in high concentrations in neurons and has been described as a surrogate biomarker of neuronal integrity and function [
67]. Total NAA (tNAA; NAA + N-acetylaspartylglutamate) in the deep gray matter was shown to be significantly lower in newborns with adverse outcomes at 18–22 months of age during the first 2 weeks after birth [
67]. In a prospective multicenter cohort study, Lally et al. demonstrated that using
1H-MRS, thalamic NAA concentration alone could accurately predict an adverse neurodevelopmental at 18–24 months of age, with a sensitivity of 100% and specificity of 97% [
14]. However, measuring absolute concentrations of metabolites is difficult, as the detection level of NAA falls in the millimolar range and requires internal or external standards. Although water-unsuppressed concentration can be used as an internal standard, this may be unreliable as the amount of intracellular and extracellular brain water may be altered by perinatal asphyxia [
69]. Metabolite ratios such as NAA/choline are often used instead and have also been demonstrated to be predictive of outcome in newborns with HIE [
70].
Lactate is a product of anaerobic metabolism, hence increased in hypoxic-ischemic conditions. It has a chemical shift of 1.33 ppm and presents as a doublet peak on spectra obtained with a short echo-time (35–40 ms) or long echo-time (272–288 ms), and an inverted peak with an echo-time around 136–144 ms (
Figure 4). Changes in lactate concentrations due to HIE are often transient; a study analyzing cerebral lactate concentrations in infants with HIE receiving therapeutic hypothermia showed high levels of lactate during hypothermia in infants with moderate-severe HIE, which slightly decreased after cooling and subsequently normalized by the end of the first week after birth [
71]. The cerebral lactate remained low in infants with no or mild brain injury during the first 2 weeks after birth. These temporal resolutions are important to take into account when interpreting
1H-MRS findings. Recently, it was demonstrated that during the first 24–96 h after birth tNAA concentration, ADC values, lactate levels, and lactate/tNAA ratios all had high prognostic value in infants with HIE undergoing therapeutic hypothermia, but only tNAA retained its good prognostic value in the second week after birth [
72]. Moreover, it is important to consider gestational age when interpreting
1H-MRS spectra. The presence of a lactate peak may be normal in preterm infants, and these infants may show relatively decreased NAA levels compared to term neonates [
73,
74].
In the past, phosphorus MRS (
31P-MRS) was used to study brain energy metabolism in human infants. Although an association between
31P-MRS findings and neurodevelopmental outcomes has been demonstrated in newborns with perinatal asphyxia, it is not routinely performed in the clinical setting [
75,
76].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12030645