Extracellular vesicles (EVs) are cargo of proteins, nucleic acids, and lipids produced by eukaryotic and prokaryotic cells both under physiological and pathological conditions. They are found in virtually all body fluids such as plasma, saliva, amniotic fluid, and breast milk, suggesting key roles in immune development and function at different life stages from in utero to aging. Under inflammatory conditions, plasma EV levels are increased and exacerbate immune activation and inflammatory reaction. During infection, bacteria derived EVs can also affect host immunity as pathogenic bacteria derived EVs having pro-inflammatory effects.
- Extracellular Vesicles
- inflammation
- immune function
- diseases
- bacteria
Communication between cells and between organs is tightly regulated and its disruption can contribute to disease development. Communication can either occur by cell-to-cell contact or by means of soluble factors. Cytokines are by definition soluble factors produced by cells (beyond immune cells) ensuring cell survival, proliferation, differentiation, and/or activation. These factors can act on the cell involved in their production (autocrine), on cells in their close environment (paracrine), or distally. Hormones are produced by endocrine organs as well as immune cells and have mostly distal effects from their sites of release. Both cytokines and hormones bind specific receptors on target cells, which then trigger a signalling activation cascade that will affect the cell phenotype. Both cytokines and hormones have been studied for decades and are the basis of modern physiology.
However, more recently, another process also involved in this type of communication has been highlighted. On top of producing specific factors, cells can send a package of information that is enclosed by cell membrane. This package is also called extracellular vesicles (EVs) and can contain DNA, RNA, proteins, sugars, and lipids. EVs can be produced by any cell type, highlighting their importance throughout evolution from unicellular organisms to metazoans. During the last decade, considerable efforts have been made to investigate the role of EVs in cell communication and disease development. In addition, numerous groups have investigated EVs for their potential as biomarkers and as targeted therapeutic carriers. However, there are major limitations in these areas that need to be addressed in the future. These limitations include a lack of standardized methodology for the isolation and purification of different classes of EVs, inadequate terminology, numerous technical challenges in EV characterization and quantification, and an incomplete identification of the cellular origin of EVs. Historically, most groups have only focused on one or two types of EVs, but have not investigated the respective contribution of all EV subclasses in their model. These are only a few major obstacles that need to be overcome to advance this field. In this review [1], we discuss the current knowledge on the production and mechanisms of action of EVs in health and disease as well as the impact of commensal and bacteria derived-EVs on cell function and their impact on health and disease.
Production of EVs by both eukaryotic and prokaryotic cells is linked to changes in both plasma and intracellular membrane homeostasis, showing that similar mechanisms for the EVs released are highly preserved throughout evolution.
Due to the fact that EVs are difficult to investigate, considerably fewer studies have focused on EVs compared to other soluble factors such as cytokines and hormones. The fact that they are important soluble factors in cell communication in both health and disease, as summarized in Figure 1, provides enormous opportunities to investigate their potential as therapeutic targets or diagnostic biomarkers.
Despite significant advances towards understanding the biology of EVs including their formation, secretion, molecular composition, and influence on the recipient cells over the past decade, the field would enormously benefit from novel protocols to more efficiently isolate the different subtypes of EVs.
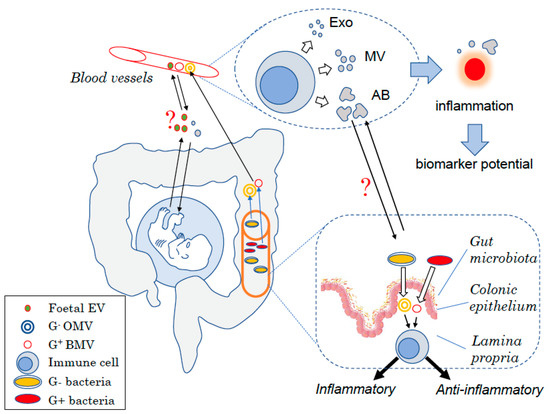
Figure 1. Schematic view of the different types of EVs in humans. EVs are produced at each body site such by mononuclear cells in the blood under the form of exosomes (Exo), microvesicles (MVs), or apoptotic bodies (AB) depending on their size. Changes in the amount of EVs produced have been linked to inflammatory diseases and thus could be used as biomarkers to diagnose diseases. EVs are also key for the host to communicate with endogenous yet “foreign” organisms. During embryonic development, the foetus produces EVs that can interact with cells from the mother and might interfere with maternal tolerance towards the haploidentical foetus. Similarly, the gut bacteria produce EVs that can modulate host–cell immune responses and more broadly the host health status. Bacterial EVs can have pro- or anti-inflammatory effects depending on the bacteria of origin. EVs may also be used for the host to communicate with these “foreign” organisms with maternal EVs that could potentially affect the foetus development as well as host EVs shown to modulate the bacteria composition.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21010107
References
- Laurence Macia; Ralph Nanan; Elham Hosseini-Beheshti; Georges E. Grau; Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development. International Journal of Molecular Sciences 2019, 21, 107, 10.3390/ijms21010107.
