A flexor tendon injury is acquired fast and is common for athletes, construction workers, and military personnel among others, treated in the emergency department. However, the healing of injured flexor tendons is stretched over a long period of up to 12 weeks, therefore, remaining a significant clinical problem. Postoperative complications, arising after traditional tendon repair strategies, include adhesion and tendon scar tissue formation, insufficient mechanical strength for early active mobilization, and infections. Various researchers have tried to develop innovative strategies for developing a polymer-based construct that minimalizes these postoperative complications, yet none are routinely used in clinical practice. Understanding the role such constructs play in tendon repair should enable a more targeted approach.
- flexor tendon repair
- anti-inflammatory
- antimicrobial
- polymer-based constructs
1. Introduction
2. New Strategies for the Repair of Flexor Tendon Injuries
2.1. Biochemical Solutions for Postoperative Complications
2.1.1. Peritendinous Adhesion Formation
2.1.2. Infections
2.2. Requirements of Polymeric Materials for Flexor Tendon Repair
- (i) Biodegradability
- (ii) Biocompatibility
- (iii) Processability and Structure Architecture
- (iv) Mechanical Properties
2.3. Materials for Flexor Tendon Scaffold and Construct Designs
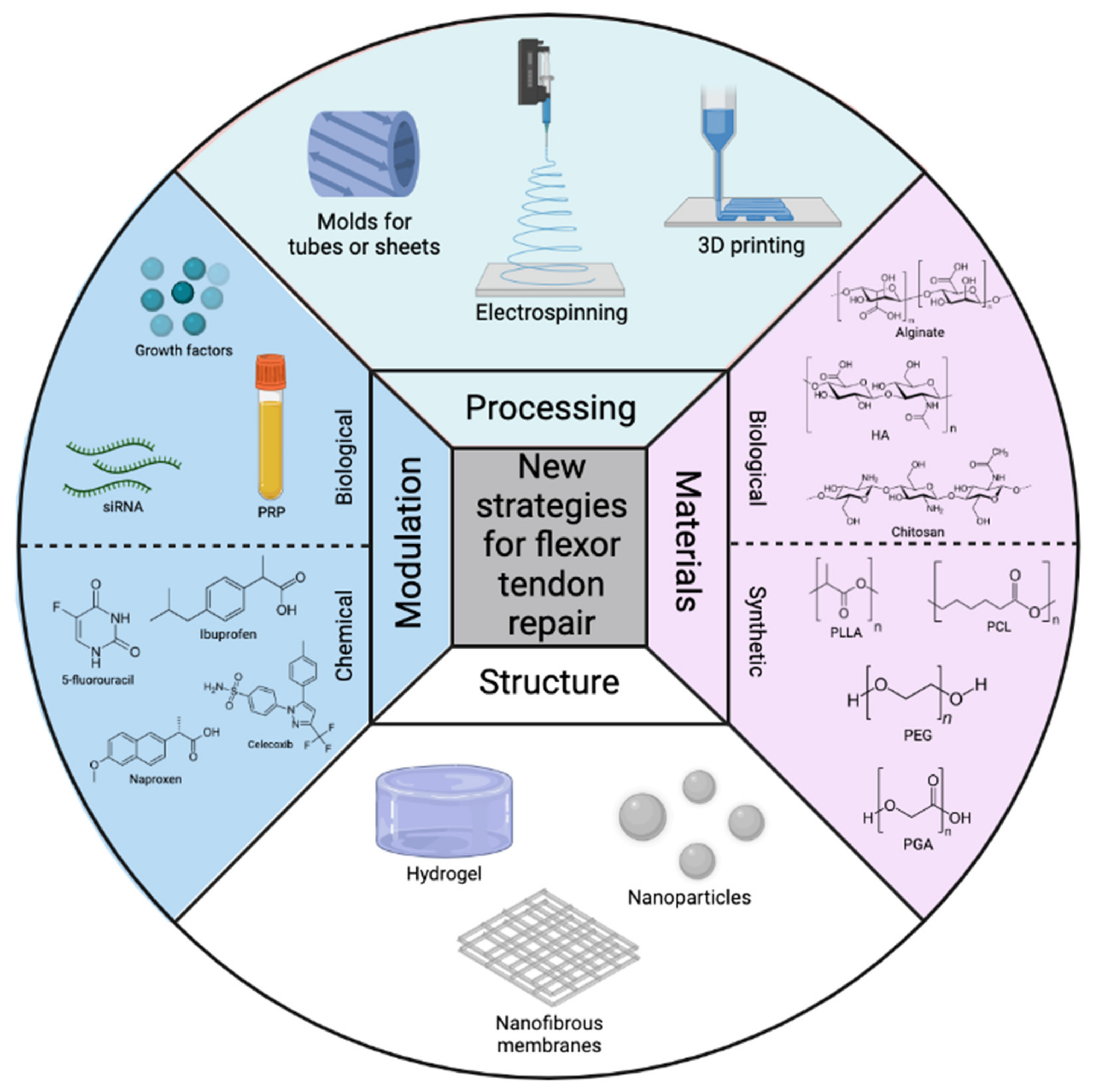 Figure 1. Overview of the most commonly used processing techniques, polymeric materials, structures, and modulations for flexor tendon repair here.
Figure 1. Overview of the most commonly used processing techniques, polymeric materials, structures, and modulations for flexor tendon repair here.3. Conclusions and Perspectives
This entry is adapted from the peer-reviewed paper 10.3390/polym14050867
References
- Kirkendall, D.T.; Garrett, W.E. Function and biomechanics of tendons. Scand. J. Med. Sci. Sport. 1997, 7, 62–66.
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36.
- Banik, B.L.; Lewis, G.S.; Brown, J.L. Multiscale Poly-(ϵ-caprolactone) Scaffold Mimicking Non-linearity in Tendon Tissue Mechanics. Regen. Eng. Transl. Med. 2016, 2, 1–9.
- Ghiya, M.N.; Murty, S.; Shetty, N.; D’Cunha, R. A descriptive study of hand injuries presenting to the adult emergency department of a tertiary care center in urban India. J. Emerg. Trauma. Shock 2017, 10, 19–25.
- Clark, D.P.; Scott, R.N.; Anderson, I.W. Hand problems in an accident and emergency department. J. Hand Surg. Br. 1985, 10, 297–299.
- Citeroni, M.R.; Ciardulli, M.C.; Russo, V.; Della Porta, G.; Mauro, A.; El Khatib, M.; Di Mattia, M.; Galesso, D.; Barbera, C.; Forsyth, N.R.; et al. Review in vitro innovation of tendon tissue engineering strategies. Int. J. Mol. Sci. 2020, 21, 1–78.
- Titan, A.L.; Foster, D.S.; Chang, J.; Longaker, M.T. Flexor Tendon: Development, Healing, Adhesion Formation, and Contributing Growth Factors. Plast. Reconstr. Surg. 2019, 144, 639e–647e.
- Legrand, A.; Kaufman, Y.; Long, C.; Fox, P.M. Molecular Biology of Flexor Tendon Healing in Relation to Reduction of Tendon Adhesions. J. Hand Surg. Am. 2017, 42, 722–726.
- Woo, S.L.Y.; Gelberman, R.H.; Cobb, N.G.; Amiel, D.; Lothringer, K.; Akeson, W.H. the importance of controlled passive mobilization on flexor tendon healing: A biomechanical study. Acta Orthop. 1981, 52, 615–622.
- Buschmann, J.; Meier Bürgisser, G. Autograft, allograft, and xenograft scaffolds for tendon and ligament repair. In Biomechanics of Tendons and Ligaments; Woodhead Publishing: Sawston, UK, 2017; ISBN 9780081004890.
- Rawson, S.; Cartmell, S.; Wong, J. Suture techniques for tendon repair; a comparative review. Muscles Ligaments Tendons J. 2013, 3, 220–228.
- Vasiliadis, A.V.; Katakalos, K. The role of scaffolds in tendon tissue engineering. J. Funct. Biomater. 2020, 11, 78.
- Sigal, I.R.; Grande, D.A.; Dines, D.M.; Dines, J.; Drakos, M. Biologic and Tissue Engineering Strategies for Tendon Repair. Regen. Eng. Transl. Med. 2016, 2, 107–125.
- Zhou, H.; Lu, H. Advances in the Development of Anti-Adhesive Biomaterials for Tendon Repair Treatment. Tissue Eng. Regen. Med. 2021, 18, 1–14.
- Beldjilali-Labro, M.; Garcia, A.G.; Farhat, F.; Bedoui, F.; Grosset, J.F.; Dufresne, M.; Legallais, C. Biomaterials in tendon and skeletal muscle tissue engineering: Current trends and challenges. Materials 2018, 11, 1116.
- Rademakers, T.; Horvath, J.M.; van Blitterswijk, C.A.; LaPointe, V.L.S. Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization. J. Tissue Eng. Regen. Med. 2019, 13, 1815–1829.
- Cravedi, P.; Farouk, S.; Angeletti, A.; Edgar, L.; Tamburrini, R.; Duisit, J.; Perin, L.; Orlando, G. Regenerative immunology: The immunological reaction to biomaterials. Transpl. Int. 2017, 30, 1199–1208.
- No, Y.J.; Castilho, M.; Ramaswamy, Y.; Zreiqat, H. Role of Biomaterials and Controlled Architecture on Tendon/Ligament Repair and Regeneration. Adv. Mater. 2020, 32, 1904511.
- Chen, S.H.; Chen, C.H.; Fong, Y.T.; Chen, J.P. Prevention of peritendinous adhesions with electrospun chitosan-grafted polycaprolactone nanofibrous membranes. Acta Biomater. 2014, 10, 4971–4982.
- Pearce, O.; Brown, M.T.; Fraser, K.; Lancerotto, L. Flexor tendon injuries: Repair & Rehabilitation. Injury 2021, 52, 2053–2067.
- Beredjiklian, P.K. Biologic aspects of flexor tendon laceration and repair. J. Bone Jt. Surg. Ser. A 2003, 85, 539–550.
- McLaughlin, R.M. Complications After Treatment of Flexor Tendon Injuries. Small Anim. Surg. Secrets Second Ed. 2004, 14, 327–330.
- Kheiran, A.; Palial, V.; Rollett, R.; Wildin, C.J.; Chatterji, U.; Singh, H.P. Cat bite: An injury not to underestimate. J. Plast. Surg. Hand Surg. 2019, 53, 341–346.
- Malizos, K.N.; Papadopoulou, Z.K.; Ziogkou, A.N.; Rigopoulos, N.; Athanaselis, E.D.; Varitimidis, S.E.; Dailiana, Z.C. Infections of Deep Hand and Wrist Compartments. Microorganisms 2020, 8, 838.
- Pickrell, B.B.; Eberlin, K.R. Secondary Surgery Following Replantation and Revascularization. Hand Clin. 2019, 35, 231–240.
- Mamane, W.; Lippmann, S.; Israel, D.; Ramdhian-Wihlm, R.; Temam, M.; Mas, V.; Pierrart, J.; Masmejean, E.H. Infectious flexor hand tenosynovitis: State of knowledge. A study of 120 cases. J. Orthop. 2018, 15, 701–706.
- Stone, J.F.; Davidson, J.S. The role of antibiotics and timing of repair in flexor tendon injuries of the hand. Ann. Plast. Surg. 1998, 40, 7–13.
- Chapman, T.; Ilyas, A.M. Pyogenic Flexor Tenosynovitis: Evaluation and Treatment Strategies. J. Hand Microsurg. 2019, 11, 121–126.
- Chan, E.; Robertson, B.F.; Johnson, S.M. Kanavel signs of flexor sheath infection: A cautionary tale. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2019, 69, 315–316.
- Trail, I.A.; Powell, E.S.; Noble, J. An evaluation of suture materials used in tendon surgery. J. Hand Surg. Br. 1989, 14, 422–427.
- Chen, C.H.; Chen, S.H.; Shalumon, K.T.; Chen, J.P. Dual functional core-sheath electrospun hyaluronic acid/polycaprolactone nanofibrous membranes embedded with silver nanoparticles for prevention of peritendinous adhesion. Acta Biomater. 2015, 26, 225–235.
- Shalumon, K.T.; Sheu, C.; Chen, C.H.; Chen, S.H.; Jose, G.; Kuo, C.Y.; Chen, J.P. Multi-functional electrospun antibacterial core-shell nanofibrous membranes for prolonged prevention of post-surgical tendon adhesion and inflammation. Acta Biomater. 2018, 72, 121–136.
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Li, C.; Hu, H.; Zhang, X. Development of silver nanoparticles loaded chitosan-alginate constructs with biomedical potentialities. Int. J. Biol. Macromol. 2017, 105, 393–400.
- Humayun, A.; Luo, Y.; Elumalai, A.; Mills, D.K. 3D printed antimicrobial PLA constructs functionalised with zinc- coated halloysite nanotubes-Ag-chitosan oligosaccharide lactate. Mater. Technol. 2022, 37, 28–35.
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 203–222.
- Khan, F.; Tanaka, M. Designing smart biomaterials for tissue engineering. Int. J. Mol. Sci. 2018, 19, 17.
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnol. 2020, 18, 23.
- Engineer, C.; Parikh, J.K.; Raval, A. Hydrolytic Degradation Behavior of Biodegradable Polymers from Controlled Drug Delivery System. Trends Biomater. Artif. Organs 2011, 25, 79–85.
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344.
- Migliaresi, C.; Fambri, L.; Cohn, D. A study on the in vitro degradation of poly(lactic acid). J. Biomater. Sci. Polym. Ed. 1994, 5, 591–606.
- Duek, E.A.R.; Zavaglia, C.A.C.; Belangero, W.D. In vitro study of poly(lactic acid) pin degradation. Polymer 1999, 40, 6465–6473.
- Jenkins, M.J.; Harrison, K.L. The effect of crystalline morphology on the degradation of polycaprolactone in a solution of phosphate buffer and lipase. Polym. Adv. Technol. 2008, 19, 1901–1906.
- Guo, M.; Chu, Z.; Yao, J.; Feng, W.; Wang, Y.; Wang, L.; Fan, Y. The effects of tensile stress on degradation of biodegradable PLGA membranes: A quantitative study. Polym. Degrad. Stab. 2016, 124, 95–100.
- Fan, Y.B.; Li, P.; Zeng, L.; Huang, X.J. Effects of mechanical load on the degradation of poly(d,l-lactic acid) foam. Polym. Degrad. Stab. 2008, 93, 677–683.
- Cooper, J.A.; Lu, H.H.; Ko, F.K.; Freeman, J.W.; Laurencin, C.T. Fiber-based tissue-engineered scaffold for ligament replacement: Design considerations and in vitro evaluation. Biomaterials 2005, 26, 1523–1532.
- Goh, J.C.; Sahoo, S. Scaffolds for tendon and ligament tissue engineering. In Regenerative Medicine and Biomaterials for the Repair of Connective Tissues; Woodhead Publishing: Sawston, UK, 2010.
- Groth, T.; Falck, P.; Miethke, R.-R. Cytotoxicity of Biomaterials—Basic Mechanisms and In Vitro Test Methods: A Review. Altern. Lab. Anim. 1995, 23, 790–799.
- Cannella, V.; Altomare, R.; Leonardi, V.; Russotto, L.; Di Bella, S.; Mira, F.; Guercio, A. In Vitro Biocompatibility Evaluation of Nine Dermal Fillers on L929 Cell Line. Biomed Res. Int. 2020, 2020, 8676343.
- Yang, S.; Leong, K.F.; Du, Z.; Chua, K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2002, 7, 679–689.
- Song, A.; Rane, A.A.; Christman, K.L. Antibacterial and cell-adhesive polypeptide and poly(ethylene glycol) hydrogel as a potential scaffold for wound healing. Acta Biomater. 2012, 8, 41–50.
- Pring, D.J.; Amis, A.A.; Coombs, R.R.H. The mechanical properties of human flexor tendons in relation to artificial tendons. J. Hand Surg. Br. Eur. Vol. 1985, 10, 331–336.
- Verdan, C.; Potenza, A.D. Tendon Surgery of the Hand. Plast. Reconstr. Surg. 1980, 66, 1493–1503.
- Tang, J.B.; Gu, Y.T.; Rice, K.; Chen, F.; Pan, C.Z. Evaluation of four methods of flexor tendon repair for postoperative active mobilization. Plast. Reconstr. Surg. 2001, 107, 742–749.
- Chen, C.T.; Chen, C.H.; Sheu, C.; Chen, J.P. Ibuprofen-loaded hyaluronic acid nanofibrous membranes for prevention of postoperative tendon adhesion through reduction of inflammation. Int. J. Mol. Sci. 2019, 20, 5038.
- Pien, N.; Peeters, I.; Deconinck, L.; Van Damme, L.; De Wilde, L.; Martens, A.; Van Vlierberghe, S.; Dubruel, P.; Mignon, A. Design and development of a reinforced tubular electrospun construct for the repair of ruptures of deep flexor tendons. Mater. Sci. Eng. C 2021, 119, 111504.
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030.
- Sensini, A.; Cristofolini, L. Biofabrication of electrospun scaffolds for the regeneration of tendons and ligaments. Materials 2018, 11, 1963.
- Narayanan, N.; Kuang, L.; Del Ponte, M.; Chain, C.; Deng, M. 1—Design and fabrication of nanocomposites for musculoskeletal tissue regeneration. In Nanocomposites for Musculoskeletal Tissue Regeneration; Liu, H., Ed.; Woodhead Publishing: Oxford, UK, 2016; pp. 3–29. ISBN 978-1-78242-452-9.
- Smith, B.D.; Grande, D.A. The current state of scaffolds for musculoskeletal regenerative applications. Nat. Rev. Rheumatol. 2015, 11, 213–222.
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926.
- Yeh, Y.-C.; Huang, T.-H.; Yang, S.-C.; Chen, C.-C.; Fang, J.-Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8, 286.
- Yoshizaki, Y.; Yuba, E.; Komatsu, T.; Udaka, K.; Harada, A.; Kono, K. Improvement of Peptide-Based Tumor Immunotherapy Using pH-Sensitive Fusogenic Polymer-Modified Liposomes. Molecules 2016, 21, 1284.
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107.
- Dong, M.; Shi, B.; Liu, D.; Liu, J.-H.; Zhao, D.; Yu, Z.-H.; Shen, X.-Q.; Gan, J.-M.; Shi, B.; Qiu, Y.; et al. Conductive Hydrogel for a Photothermal-Responsive Stretchable Artificial Nerve and Coalescing with a Damaged Peripheral Nerve. ACS Nano 2020, 14, 16565–16575.
- Zhang, K.; Lv, H.; Zheng, Y.; Yao, Y.; Li, X.; Yu, J.; Ding, B. Nanofibrous hydrogels embedded with phase-change materials: Temperature-responsive dressings for accelerating skin wound healing. Compos. Commun. 2021, 25, 100752.
