1. Introduction
Presbyopia is a physiologically age-related reduction of accommodation leading to unsatisfied clarity of the near vision [
1]. This condition usually starts after age of 45. In 2015, it was estimated that approximately 1.8 billion people were affected by presbyopia globally which was about 25% of the world population, and approximately 826 million people lacked proper visual correction. In the year 2030, the number of people with presbyopia is expected to be increasing to 2.1 billion people globally [
2].
Accommodation is a mechanism enabling eyes to adjust their refraction power to focus on near objects. There are three main processes involved in accommodation. They are (1) ciliary muscle contraction, which in turn reduces the zonular tension and results in increased lens thickness, (2) pupillary constriction, and (3) convergence of both eyes [
3,
4]. The widely accepted cause of presbyopia is the stiffening of the lens, which limits lens thickening [
5].
Presbyopia does not only affect the near vision, which is the distance between 20 and 40 cm from the eyes, but also affects the intermediate vision, which is the distance between 50 to 100 cm from the eyes [
6].
Treatment and correction of presbyopia are still challenging since there are no drugs or procedures that can cause perfect vision at all distances without risk. Currently, there are several options to treat presbyopia: optical correction, including bifocal or progressive spectacles, monofocal or multifocal contact lenses, corneal or intraocular surgical procedures, and pharmacological treatment.
For optical correction with spectacles, such as monofocal, bifocal or multifocal lenses, they are common options because of easy access and non-invasive approach. However, spectacles are perceived by many patients as inconvenient [
7,
8,
9]. Monovision contact lenses may deteriorate stereopsis since the lens is put on only one eye for near tasks. When there is a difference between focusing power of both eyes, the depth discrimination is affected [
10]. Monovision associated with anisometropia of +2.00 diopters or higher may decrease stereoacuity [
11]. Multifocal contact lenses may be an alternative to spectacles, however, they may cause discomfort, or inconvenience for some patients, particularly those who have never worn contact lens [
12]. Contact lenses may also be related with a risk of serious ocular surface infections [
13].
Surgical options, corneal or intraocular, are of increasing interest since they are based on most modern technologies. Corneal surgery, such as corneal monovision, corneal inlays, collagen shrinkage, or multifocal LASIK, was one of the common methods for presbyopia correction. They have shown successes in improving near vision, however, there are disadvantages, such as, reduction of intermediate or distance vision, decreased contrast sensitivity, dysphotopsia, or refractive regression. Due to these, some patients still need spectacles after the procedures [
1,
14]. Intraocular lenses (IOLs), such as monovision IOLs or multifocal IOLs, were also used for correction of presbyopia, with disadvantages, such as, dysphotopsia, or poorer intermediate vision, similar to corneal surgeries [
14,
15].
There are still risks of surgical complications, which are hardly reversible, and the best results are based on careful selection of patients. Moreover, good understanding of the limitation of the present technologies by patients and their trade-off nature are very important, for example, patients who gain spectacle-free of near or intermediate vision may experience some dysphotopsias in different lighting conditions or lose some sharpness of vision or stereopsis depending on the offered technology [
16]. Finally, none of the present surgical technologies can offer full spectacles independence for the whole time of all activities.
Pharmacological treatment of presbyopia has been studied in recent years based on different drugs and different treatment regimens. Pharmacological treatment, in theory, may offer a benefit of having a spectacle-free condition with a lower risk of irreversible ocular complications, compared to surgery. In November 2021, U.S. FDA has approved 1.25% pilocarpine hydrochloride ophthalmic solution (AGN-190584) as an eye drop for treating presbyopia [
17]. This is the first eye drop treatment of presbyopia that obtained U.S. FDA approval. It is possible that this approval may cause more interest in research on pharmacological treatment for presbyopia. On the other hand, there will be more data on efficacy and safety of the drug from the real-world experience, which may lead to better understanding of presbyopia.
2. Pharmacological Treatment in Presbyopia
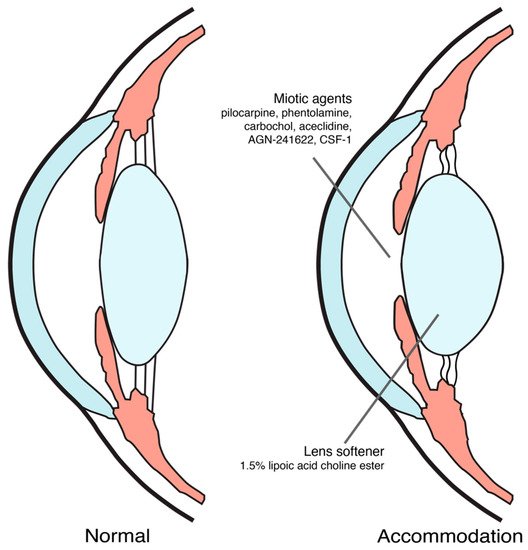
Until now, proposed mechanisms of action for pharmacological treatment of presbyopia were inducing miosis and softening the lens [9,18,19], as shown in Figure 1. The ongoing trials on pharmacological treatment of presbyopia were summarized in Table 1.
Figure 1. Pharmacological treatment for presbyopia mechanism of action.
Table 1. Ongoing studies on pharmacological treatment for presbyopia from clinicaltrials.gov (at the time of writing).
| Drugs |
N |
Study Design |
Instillation Method |
Primary Outcome |
NCT ID |
Phase |
| |
Miotic agents |
|
| 1.25% pilocarpine [20] |
230 |
Multi-center, double-masked, randomized, vehicle-controlled, parallel-group study |
Twice daily binocularly for 14 days |
Percentage of participants gaining 3 lines or more in mesopic, high contrast, binocular DCNVA at day 14 |
NCT04983589 |
3 |
| 1.25% pilocarpine [21] |
54 |
Randomized, double-masked, crossover study |
Twice daily binocularly for 14 days |
Overall Composite Driving Z score approximately 1 h after study intervention instillation |
NCT04837482 |
3 |
| AGN-241622 [22] |
144 |
Phase 1/2, dose-ascending, multi-center, randomized, double-masked, vehicle-controlled study |
Single drop binocularly |
Number of patients experiencing a treatment emergent adverse event after single administration of AGN-241622 at day 2 and day 14 |
NCT04403763 |
1/2 |
| CSF-1 [23] |
300 |
4-visit, multi-center, randomized, double-masked, vehicle-controlled study |
Twice daily binocularly for 2 weeks |
Percentage of subjects with a ≥ 3-line gain in BDCVA at 40 cm and no loss in BDCVA ≥ 5 letters at 4 m at day 8 |
NCT04599933 |
3 |
| CSF-1 [24] |
300 |
4-visit, multi-center, randomized, double-masked, vehicle-controlled study |
Twice daily binocularly for 2 weeks |
Percentage of subjects with a ≥ 3-line gain in BDCVA at 40 cm and no loss in BDCVA ≥ 5 letters at 4 m at day 8 |
NCT04599972 |
3 |
| 1% phentolamine [25] |
150 |
Randomized, quadruple-masked, parallel-group study |
Single drop binocularly |
Percentage of subjects with ≥15 letters of improvement in photopic binocular DCNVA after 6 h |
NCT04675151 |
2 |
| Carbachol and brimonidine [26] |
450 |
Multi-center, randomized, double-masked study |
Single drop binocularly |
Percentage of subjects with 3-line gains in near VA with the loss of at least 1 line in DVA |
NCT05135286 |
3 |
| PBOHB compound [27] |
11 |
Single group study |
Single drop binocularly |
Jaeger near uncorrected visual acuity improvement after 1 h |
NCT05006911 |
1 |
| Pilocarpine cream [28] |
120 |
Multi-center, randomized, double-masked, placebo-controlled, parallel group study |
Once daily binocularly for 28 days |
Binocular DCNVA after 28 days |
NCT05124275 |
2 |
| Pilocarpine Spray [29] |
139 |
Randomized, triple-masked, crossover, placebo-controlled study |
Single drop binocularly |
Proportion of subjects gaining ≥ 15 letters in mesopic, high contrast, binocular DCNVA at 120 min post-treatment |
NCT05114486 |
3 |
| |
Lens softeners |
|
| 1.5% lipoic acid choline ester [30] |
225 |
Multi-center, randomized, placebo-controlled, double-masked, dose-ranging study |
Twice daily binocularly |
Change in Binocular DNCVA From Baseline at Month 3 |
NCT04806503 |
2 |