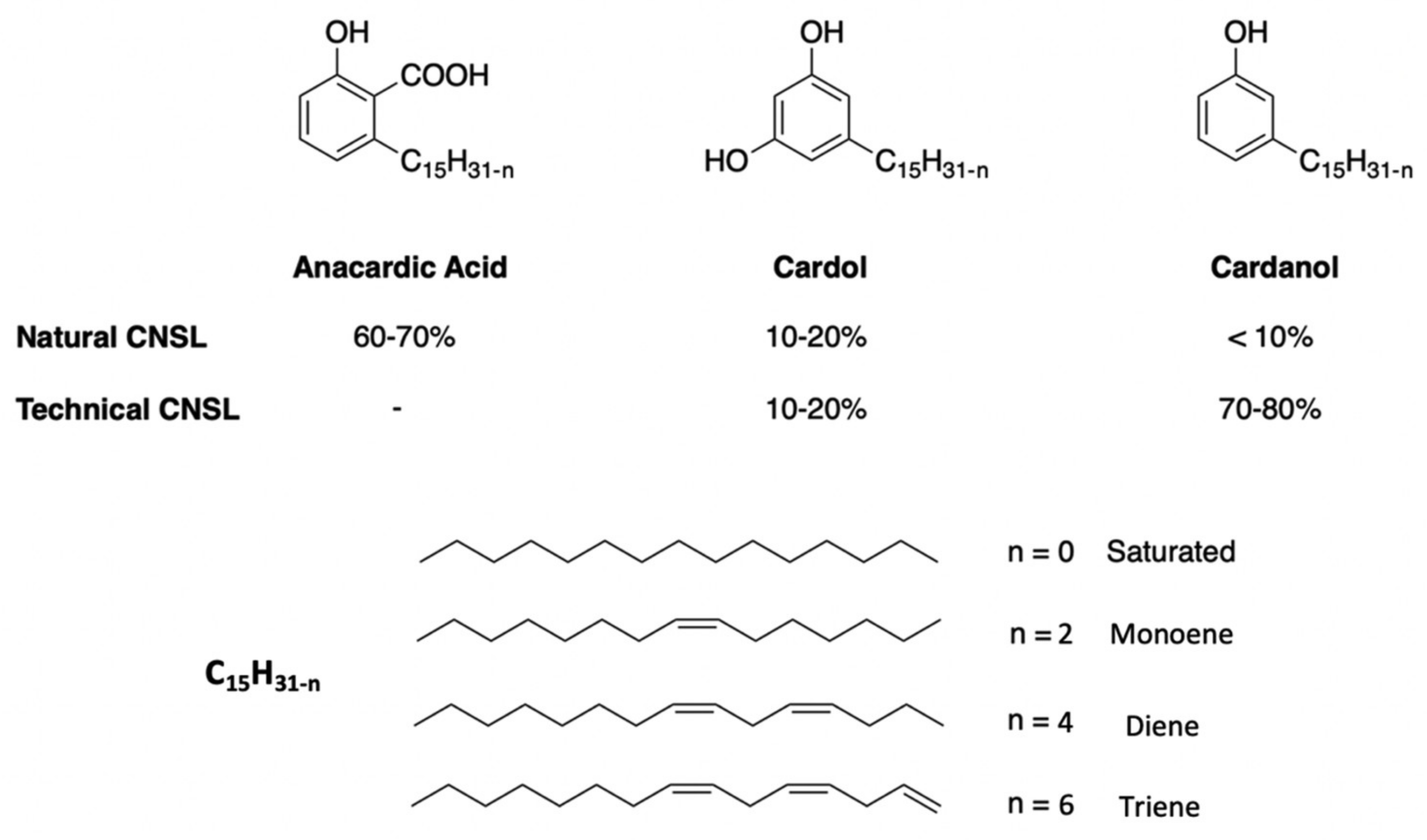
Cashew Nut Shell Liquid (CNSL) is a promising non-edible renewable resource, directly extracted from the shell of the cashew nut. The interesting structure of CNSL and its components (cardanol, anacardic acid and cardol) lead to the synthesis of biobased surfactants.
- CNSL
- cardanol
- surfactant
- biobased
1. Introduction
Numerous studies are bringing to light the possible toxicity of currently widely used products such as pesticides [1], and additives [2][3][4]. This has the effect of accelerating the search for alternatives that are as safe as possible for human beings and the planet on which human live. It is in this momentum that sustainable and green chemistry is emerging and defined [5][6]. The aim and the definition of sustainability are to reconcile economic, environmental, and social dimensions with the aid of the 3Ps: People, Planet, and Profit, around the same objective, and to ensure its continuity over time [7][8].
In these times, when components and additives are deeply studied, scrutinized, and criticized, surfactants are crystallizing a certain focus for consumer interest. Present in the shampoos, detergents, household products, cosmetics, and also in the world of construction and materials, their omnipresence and their possible impact are worrying. Their proximity to human body, the food, and the water people consume make surfactants a potential source of pollution and danger for humans, but also for rivers, oceans, sea life, wildlife, and flora on which people depend, and for which people are responsible [9][10][11].
2. Cashew NutShell Liquid
3. Synthesis of CNSL-Based Surfactants
3.1. Introduction
3.2. Anionic Surfactants Based on CNSL
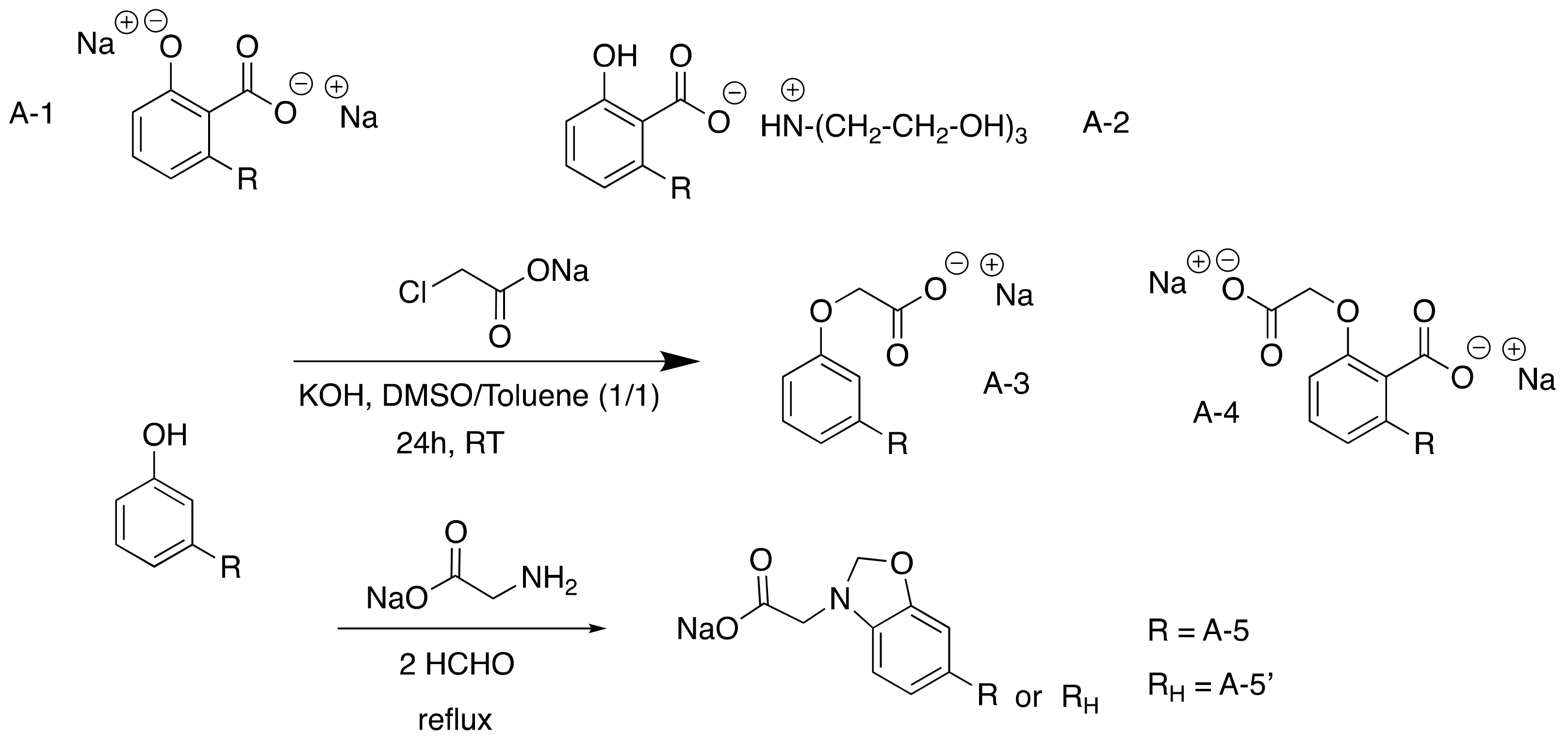
3.2.1. Carboxylated CNSL-Based Surfactants
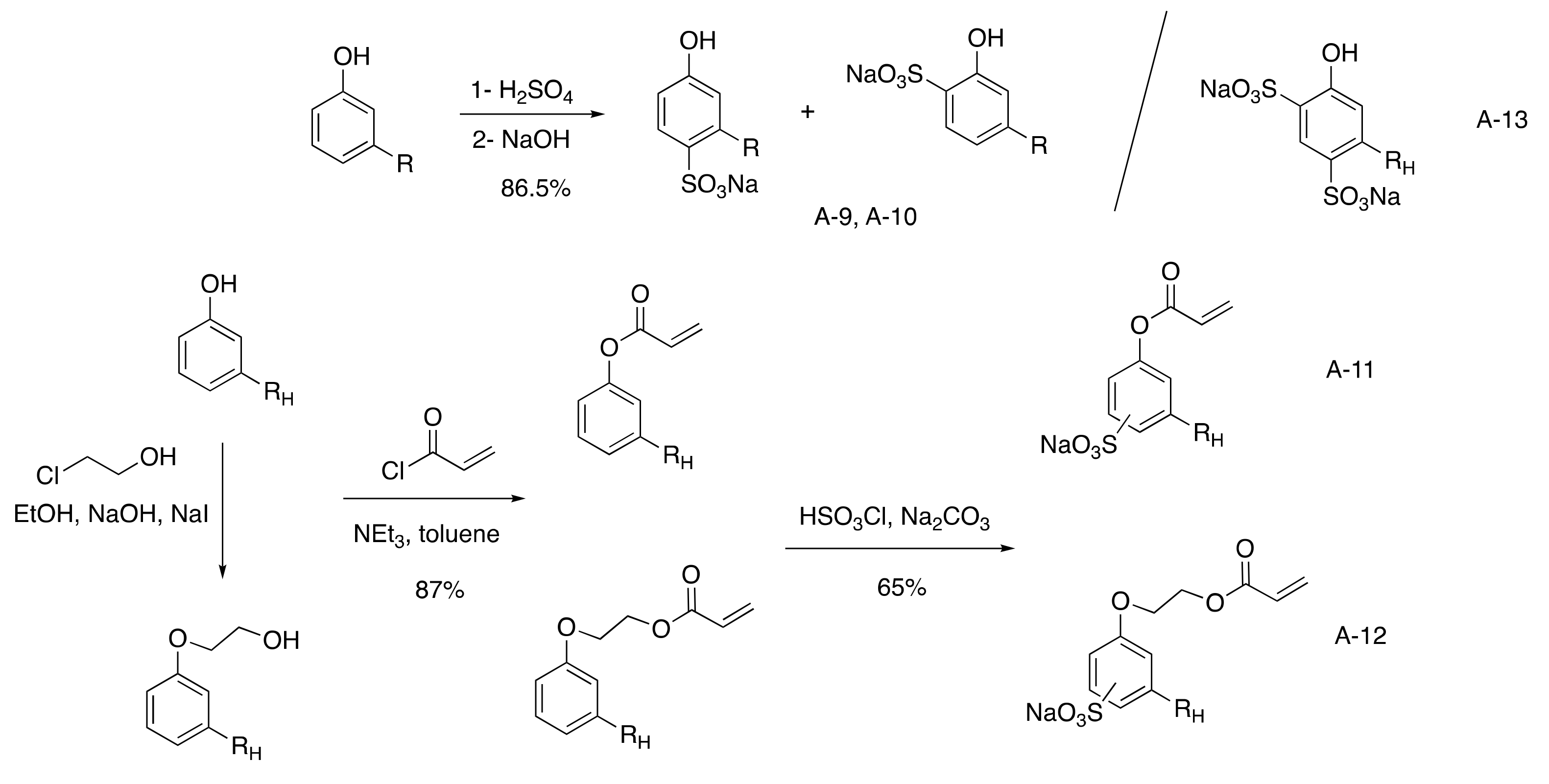
3.2.2. Sulfonated CNSL-Based Surfactants
Aromatic Sulfonated Surfactants
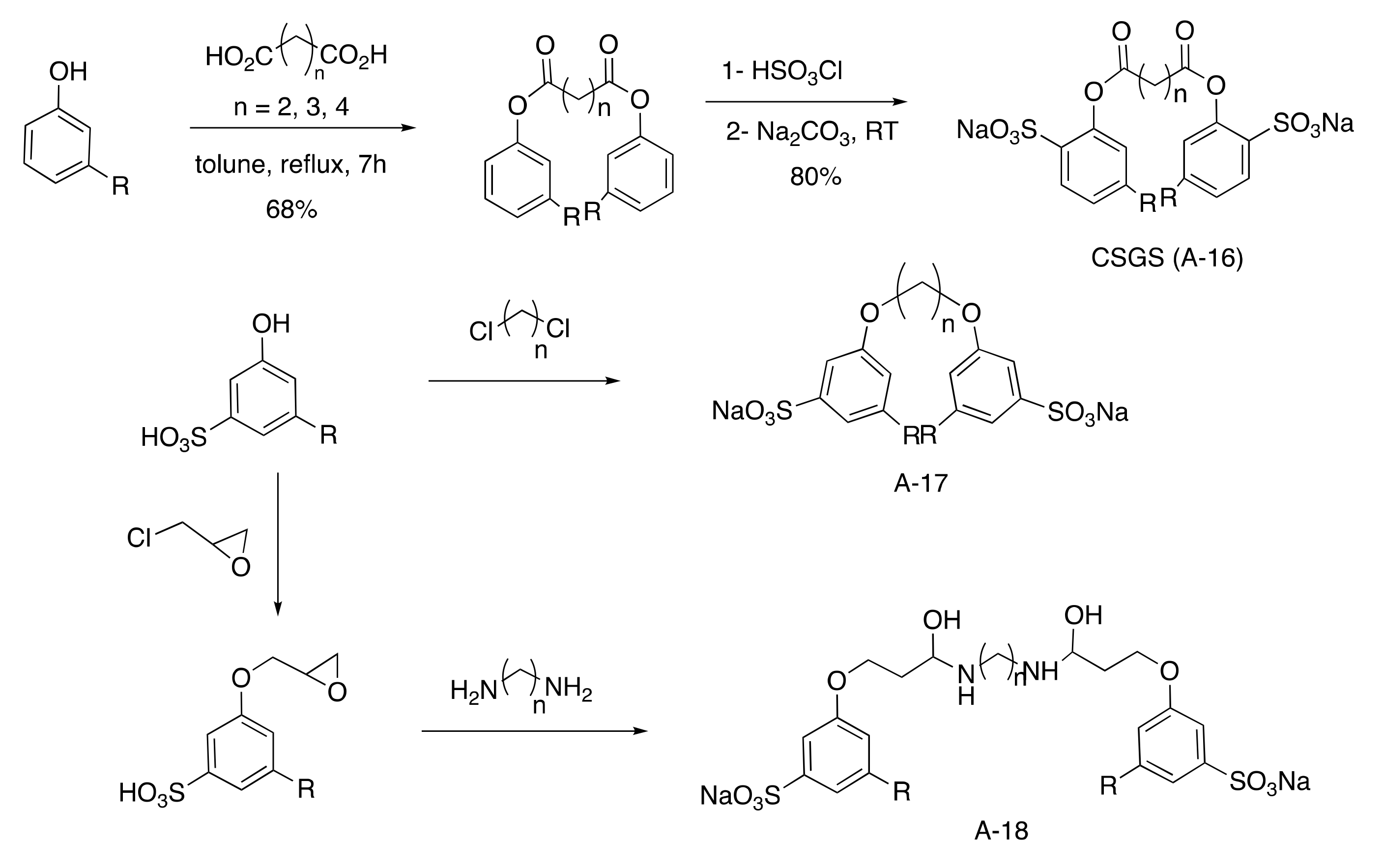
Gemini Aromatic Sulfonated Surfactants
3.3. Cationic Surfactants Based on CNSL
3.3.1. Ammonium Derivatives of Cardanol
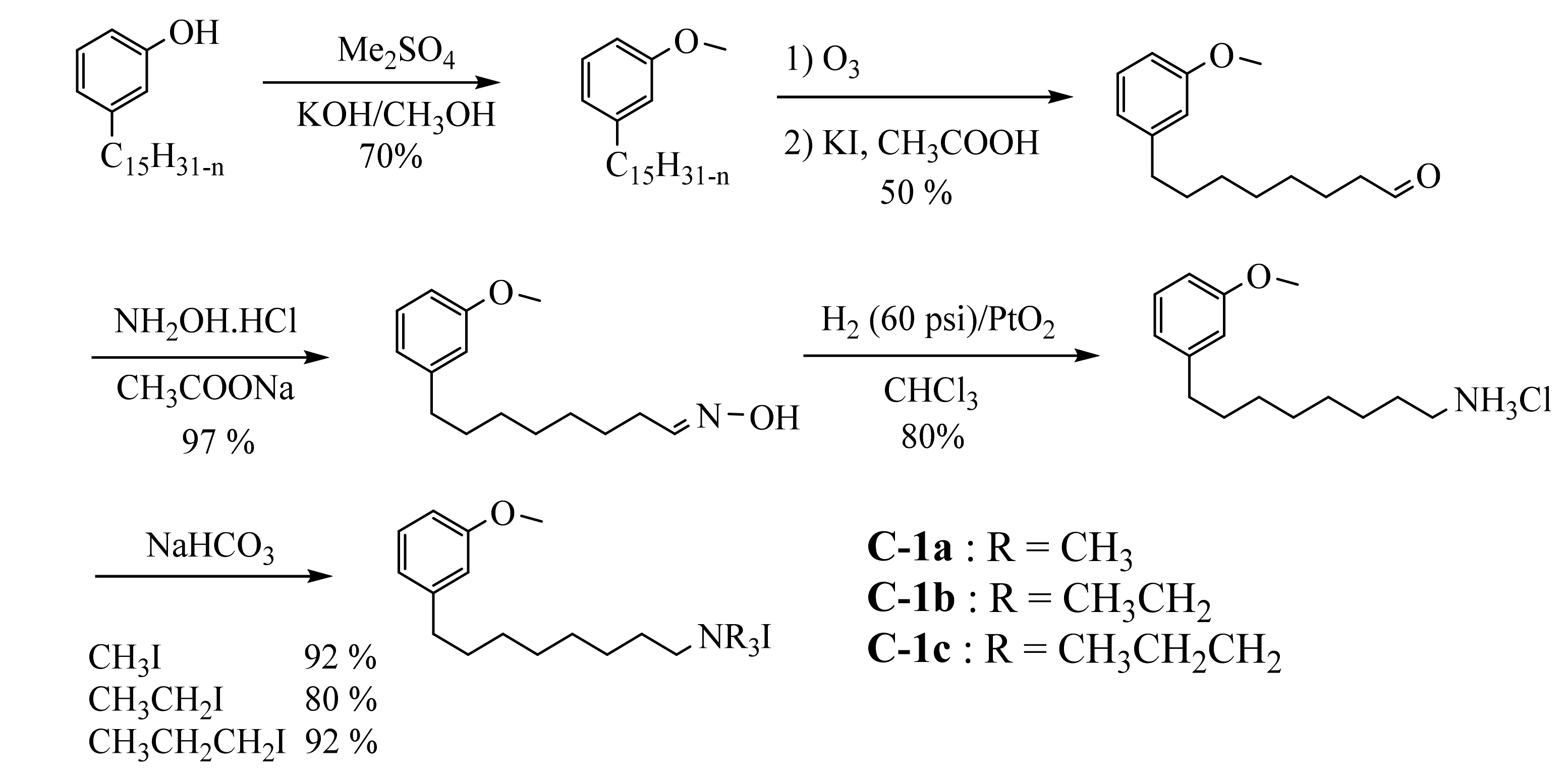
3.3.2. Macromolecular Ammonium CNSL-Based Surfactants
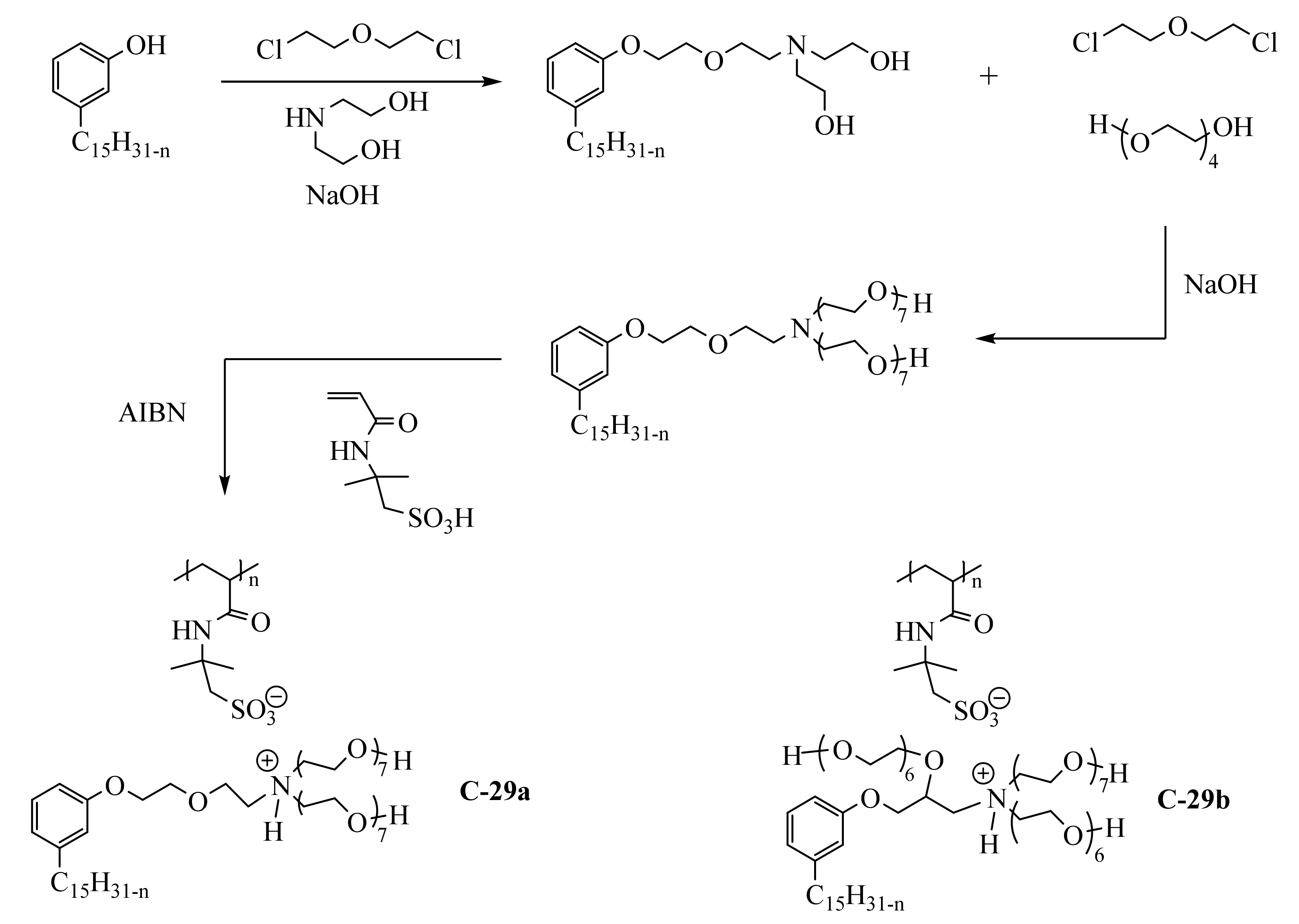
3.3.3. Pyridinium Derivatives of Cardanol
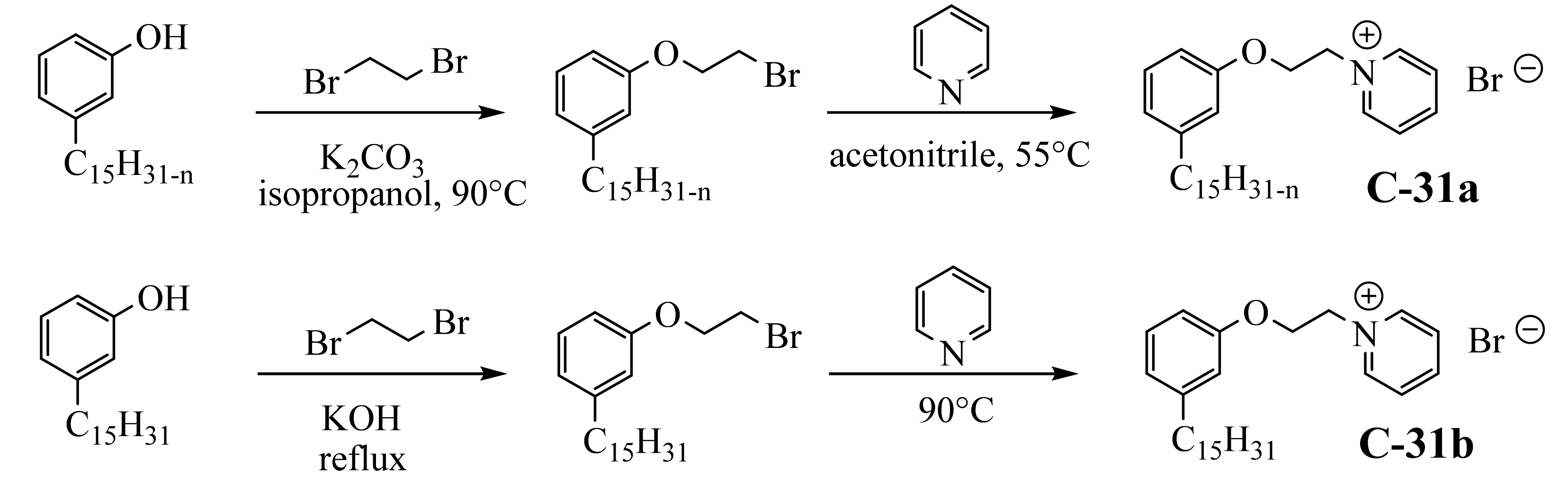
3.4. Zwitterionic Surfactants Based on CNSL
3.5. Nonionic Surfactants Based on CNSL
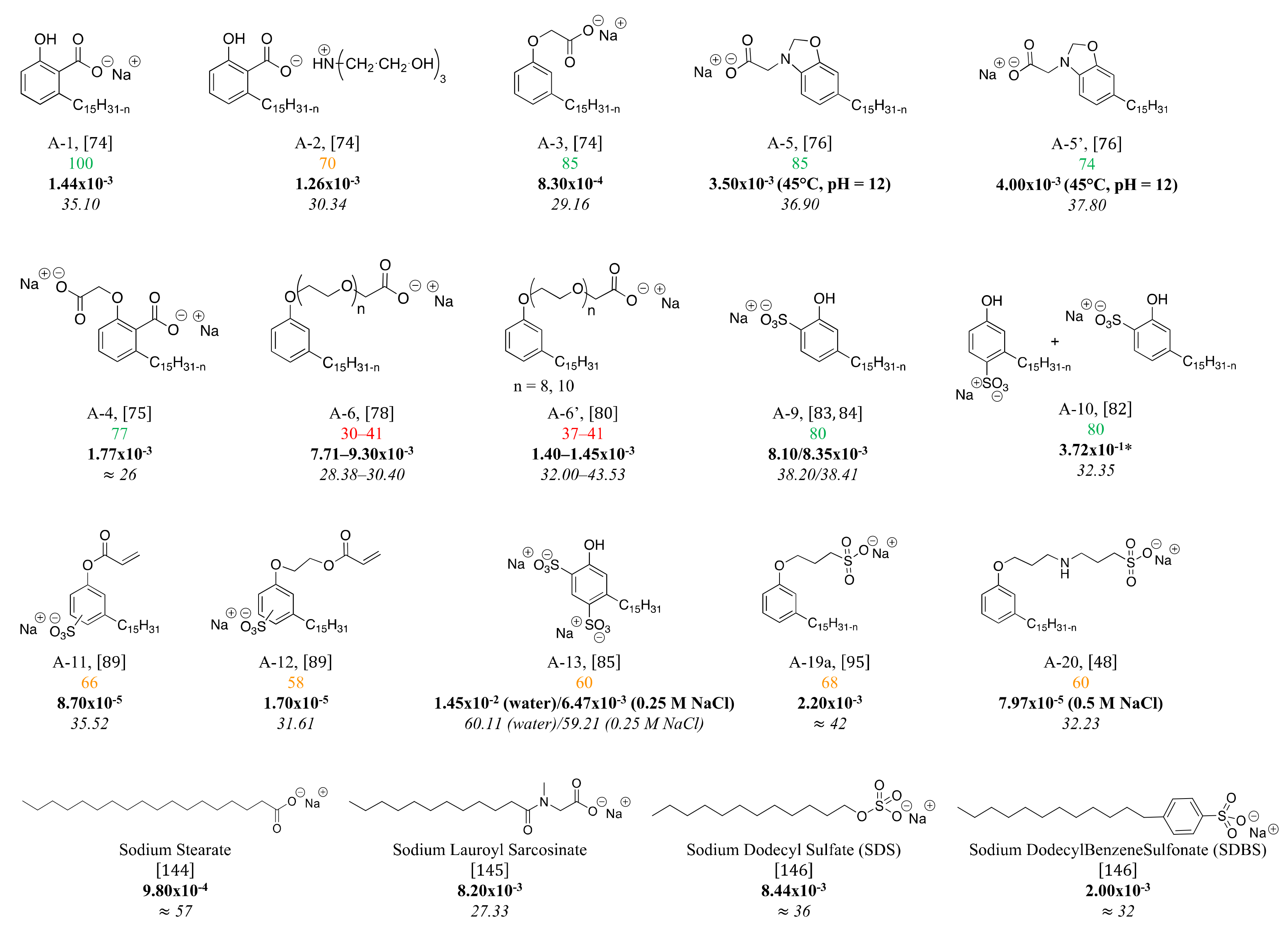
4. Structure-Property Relationship for CNSL-Based Surfactants
Indeed, CNSL surfactants are characterized by a large structural diversity, whether in terms of the nature of the polar head (anionic, cationic, zwitterionic, or nonionic) and/or the hydrophobic tail into the cardanol, cardol, or anacardic acid with the presence of alkenes on the C15 side chain combined with other hydrophilic/hydrophobic groups (alcohol, ether, amine, etc.). For example, several publications reported the preparation of Gemini (or dimeric) surfactants, which are composed of two polar heads and two hydrophobic tails, linked together by a spacer [54][55]. Gemini surfactants are known to be considerably more surface-active than conventional (single chain) surfactants.
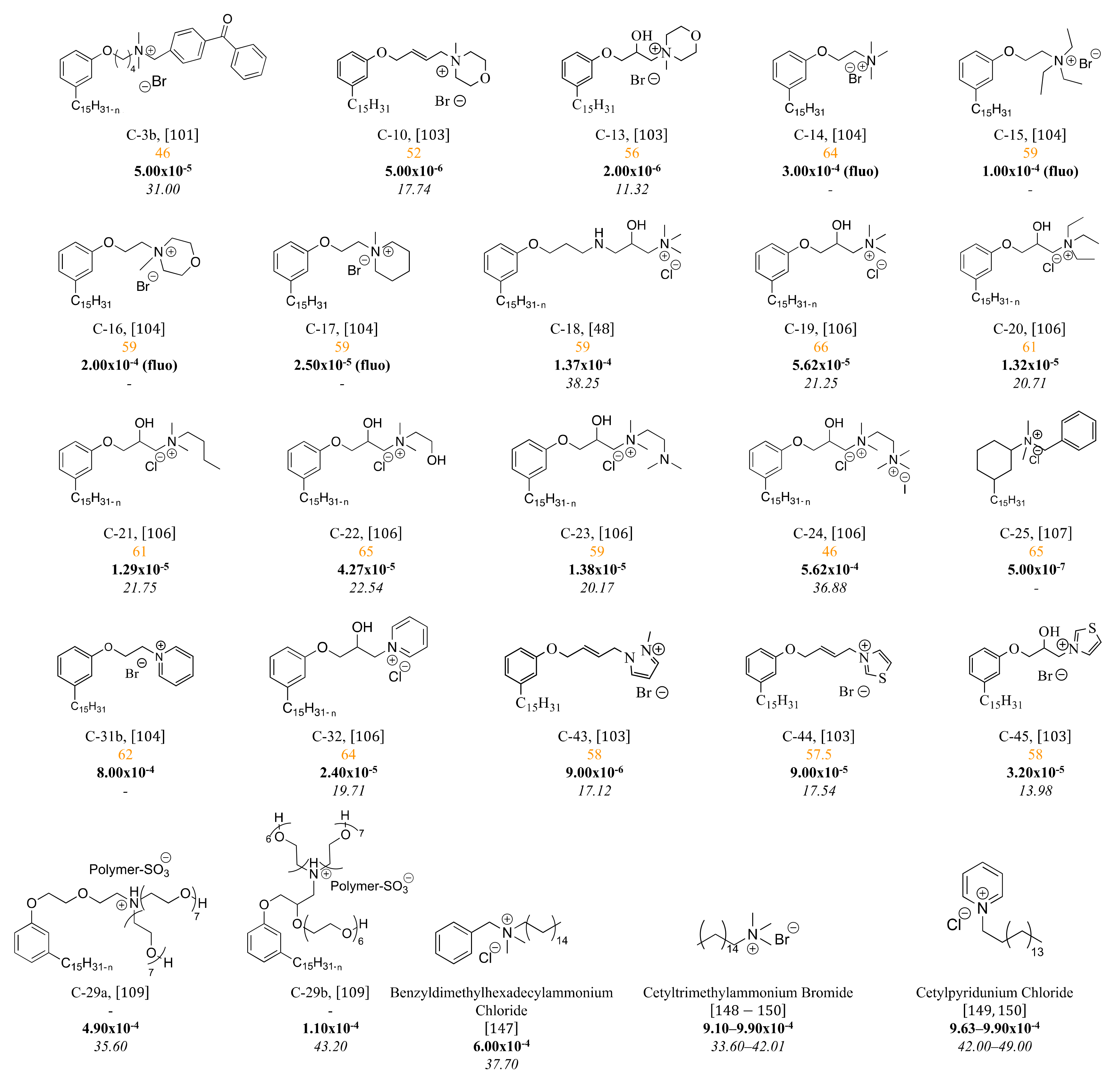
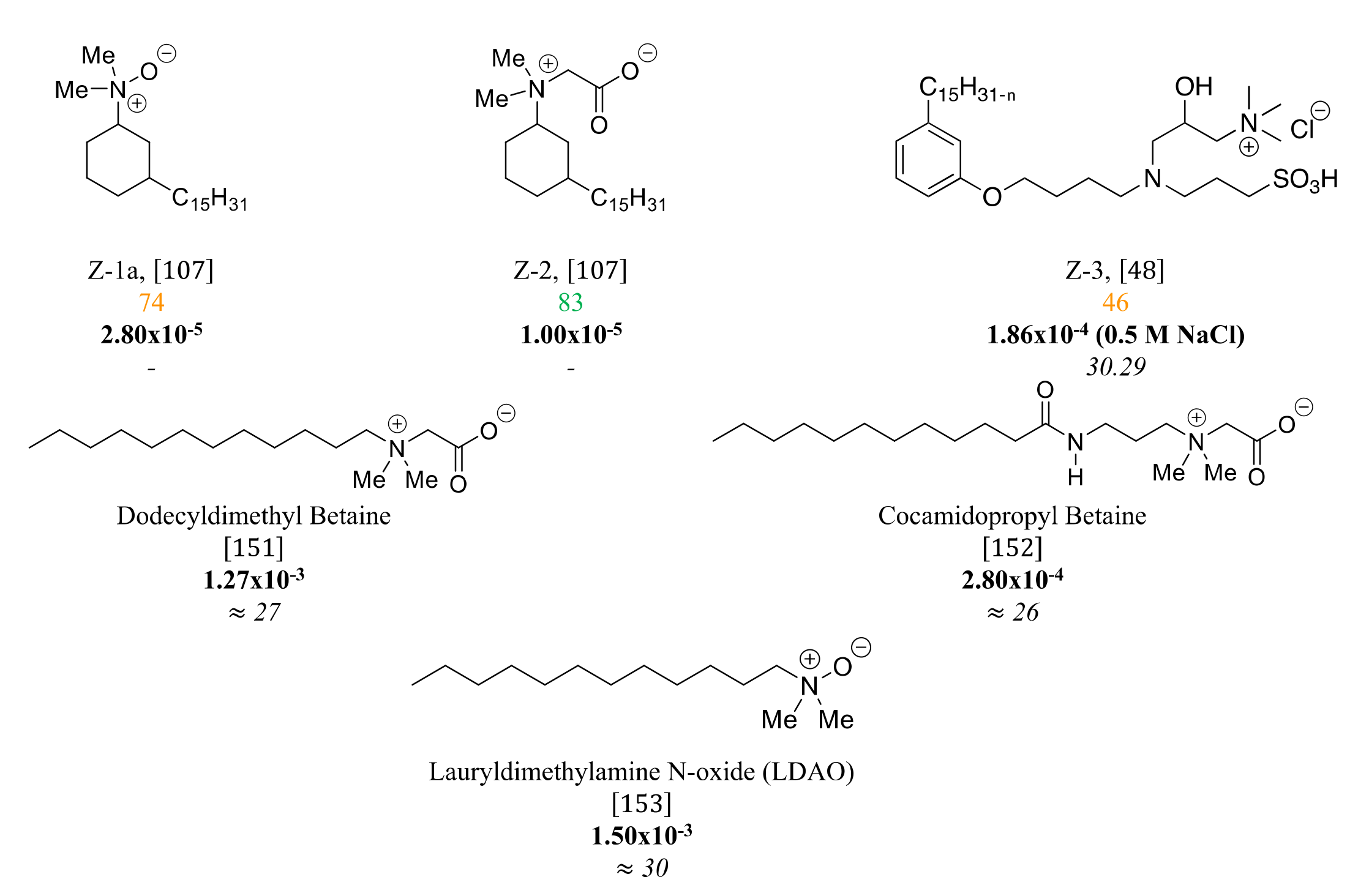
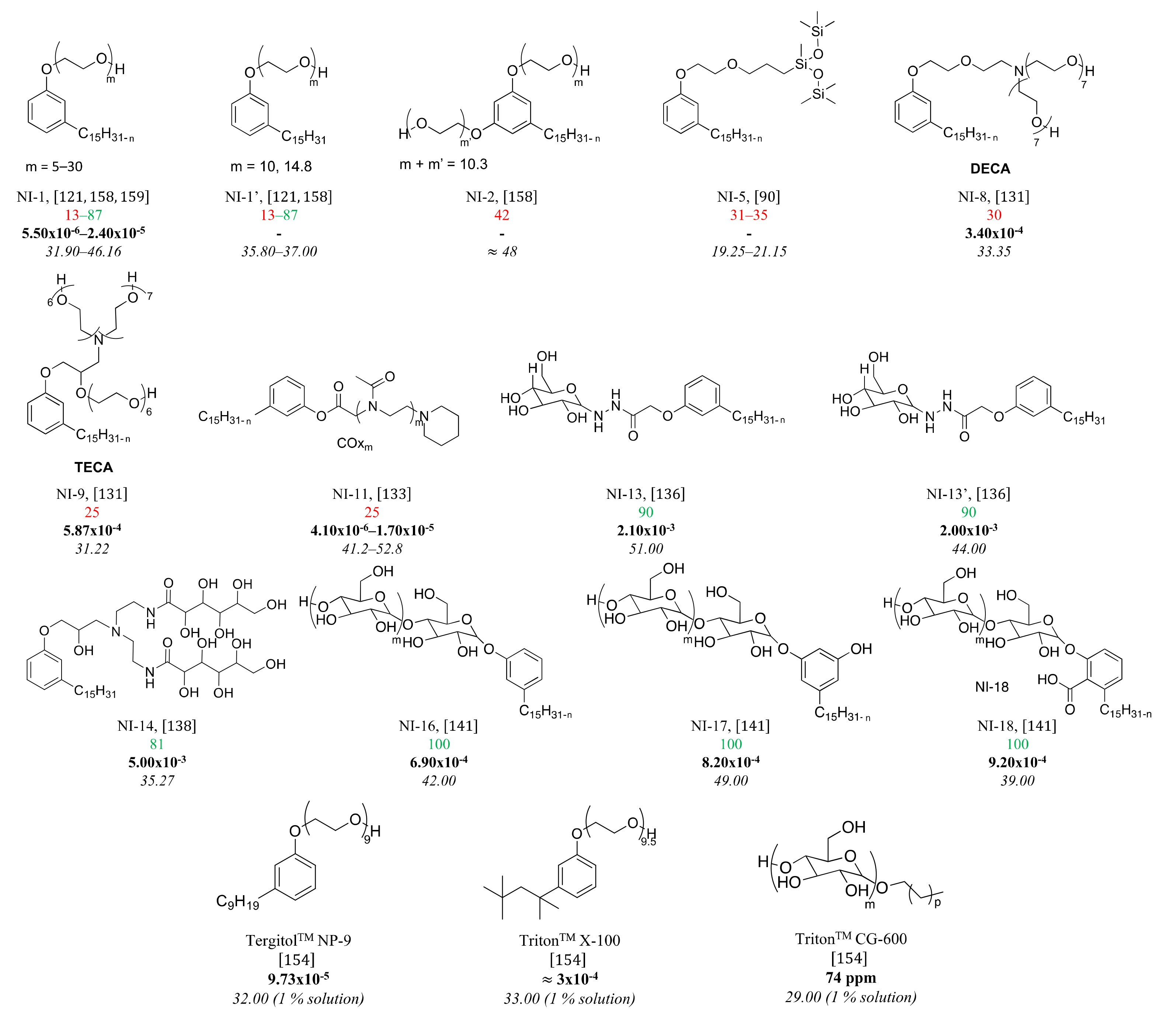
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/molecules27041443
References
- Mesnage, R.; Séralini, G.-E. Editorial: Toxicity of Pesticides on Health and Environment. Front. Public Health 2018, 6.
- Bolívar-Subirats, G.; Rivetti, C.; Cortina-Puig, M.; Barata, C.; Lacorte, S. Occurrence, toxicity and risk assessment of plastic additives in Besos river, Spain. Chemosphere 2021, 263, 128022.
- Baralić, K.; Buha Djordjevic, A.; Živančević, K.; Antonijević, E.; Anđelković, M.; Javorac, D.; Ćurčić, M.; Bulat, Z.; Antonijević, B.; Đukić-Ćosić, D. Toxic Effects of the Mixture of Phthalates and Bisphenol A—Subacute Oral Toxicity Study in Wistar Rats. Int. J. Environ. Res. Public Health 2020, 17, 746.
- Naveira, C.; Rodrigues, N.; Santos, F.S.; Santos, L.N.; Neves, R.A.F. Acute toxicity of Bisphenol A (BPA) to tropical marine and estuarine species from different trophic groups. Environ. Pollut. 2021, 268, 115911.
- Horváth, I.T. Introduction: Sustainable Chemistry. Chem. Rev. 2018, 118, 369–371.
- Anastas, P.T.; Warner, J.C. Green chemistry. Frontiers 1998, 640, 1998.
- Brown, B.J.; Hanson, M.E.; Liverman, D.M.; Merideth, R.W. Global sustainability: Toward definition. Environ. Manag. 1987, 11, 713–719.
- Marion, P.; Bernela, B.; Piccirilli, A.; Estrine, B.; Patouillard, N.; Guilbot, J.; Jérôme, F. Sustainable chemistry: How to produce better and more from less? Green Chem. 2017, 19, 4973–4989.
- Lewis, M.A. Chronic and sublethal toxicities of surfactants to aquatic animals: A review and risk assessment. Water Res. 1991, 25, 101–113.
- Drobeck, H. Current Topics on the Toxicity of Cationic Surfactants. In Cationic Surfactants; CRC Press: Boca Raton, FL, USA, 1994; p. 34.
- Attwood, D.; Florence, A.T. Aspects of surfactant toxicity. In Surfactant Systems: Their Chemistry, Pharmacy and Biology; Attwood, D., Florence, A.T., Eds.; Springer: Dordrecht, The Netherlands, 1983; pp. 614–697.
- Stubenrauch, C. Sugar surfactants—Aggregation, interfacial, and adsorption phenomena. Curr. Opin. Colloid Interface Sci. 2001, 6, 160–170.
- Vafakish, B.; Wilson, L.D. A Review on Recent Progress of Glycan-Based Surfactant Micelles as Nanoreactor Systems for Chemical Synthesis Applications. Polysaccharides 2021, 2, 12.
- Valerio, O.; Misra, M.; Mohanty, A.K. Poly(glycerol-co-diacids) Polyesters: From Glycerol Biorefinery to Sustainable Engineering Applications, A Review. ACS Sustain. Chem. Eng. 2018, 6, 5681–5693.
- Sutter, M.; Silva, E.D.; Duguet, N.; Raoul, Y.; Métay, E.; Lemaire, M. Glycerol Ether Synthesis: A Bench Test for Green Chemistry Concepts and Technologies. Chem. Rev. 2015, 115, 8609–8651.
- Alwadani, N.; Fatehi, P. Synthetic and lignin-based surfactants: Challenges and opportunities. Carbon Resour. Convers. 2018, 1, 126–138.
- Christen, V.; Mittner, F.; Fent, K. Molecular Effects of Neonicotinoids in Honey Bees (Apis mellifera). Environ. Sci. Technol. 2016, 50, 4071–4081.
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395.
- Wang, L.; Queneau, Y. Carbohydrate-Based Amphiphiles: Resource for Bio-based Surfactants. In Green Chemistry and Chemical Engineering; Han, B., Wu, T., Eds.; Springer: New York, NY, USA, 2019; pp. 349–383.
- Bhadani, A.; Kafle, A.; Ogura, T.; Akamatsu, M.; Sakai, K.; Sakai, H.; Abe, M. Current perspective of sustainable surfactants based on renewable building blocks. Curr. Opin. Colloid Interface Sci. 2020, 45, 124–135.
- Foley, P.; Beach, E.S.; Zimmerman, J.B. Derivation and synthesis of renewable surfactants. Chem. Soc. Rev. 2012, 41, 1499–1518.
- Yang, X.H.; Wang, Z.M.; Jing, F.; Hu, L.H.; Zhou, Y.H. A Brief Review of Cardanol-Based Surfactants. Appl. Mech. Mater. 2014, 483, 83–87.
- Caillol, S. Cardanol: A promising building block for biobased polymers and additives. Curr. Opin. Green Sustain. Chem. 2018, 14, 26–32.
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162.
- Ábrego, J.; Plaza, D.; Luño, F.; Atienza-Martínez, M.; Gea, G. Pyrolysis of cashew nutshells: Characterization of products and energy balance. Energy 2018, 158, 72–80.
- Senthil Kumar, P.; Arun Kumar, N.; Sivakumar, R.; Kaushik, C. Experimentation on solvent extraction of polyphenols from natural waste. J. Mater. Sci. 2009, 44, 5894–5899.
- Chaudhari, A.P.; Thakor, N.J. Extraction of CNSL usign screw pressing. Asian J. Biol. Life Sci. 2015, 4, 71–75.
- Shobha, S.V.; Ravindranath, B. Supercritical carbon dioxide and solvent extraction of the phenolic lipids of cashew nut (Anacardium occidentale) shells. J. Agric. Food Chem. 1991, 39, 2214–2217.
- Patel, R.N.; Bandyopadhyay, S.; Ganesh, A. Extraction of cashew (Anacardium occidentale) nut shell liquid using supercritical carbon dioxide. Bioresour. Technol. 2006, 97, 847–853.
- Chatterjee, S.; Dhanurdhar; Rokhum, L. Extraction of a cardanol based liquid bio-fuel from waste natural resource and decarboxylation using a silver-based catalyst. Renew. Sustain. Energy Rev. 2017, 72, 560–564.
- Paramashivappa, R.; Kumar, P.P.; Vithayathil, P.J.; Rao, A.S. Novel Method for Isolation of Major Phenolic Constituents from Cashew (Anacardium occidentale L.) Nut Shell Liquid. J. Agric. Food Chem. 2001, 49, 2548–2551.
- Durrani, A.A.; Davis, G.L.; Sood, S.K.; Tychopoulos, V.; Tyman, J.H.P. Long-chain phenols. Part 231 practical separations of the component phenols in technical cashew nut-shell liquid (Anacardium occidentale): Distillation procedures for obtaining cardanol. J. Chem. Technol. Biotechnol. 1982, 32, 681–690.
- Phani Kumar, P.; Paramashivappa, R.; Vithayathil, P.J.; Subba Rao, P.V.; Srinivasa Rao, A. Process for Isolation of Cardanol from Technical Cashew (Anacardium occidentale L.) Nut Shell Liquid. J. Agric. Food Chem. 2002, 50, 4705–4708.
- Yuliana, M.; Nguyen-Thi, B.T.; Faika, S.; Huynh, L.H.; Soetaredjo, F.E.; Ju, Y.-H. Separation and purification of cardol, cardanol and anacardic acid from cashew (Anacardium occidentale L.) nut-shell liquid using a simple two-step column chromatography. J. Taiwan Inst. Chem. Eng. 2014, 45, 2187–2193.
- Surfactants Market by Type (Anionic, Non-ionic, Cationic, Amphoteric, and Others) and Application (Household Detergents, Personal Care, Industrial & Institutional Cleaners, Food Processing, Oilfield Chemicals, Agricultural Chemicals, Textiles, Emulsion Polymerization (Plastics), Paints & Coatings, Construction, and Others): Global Opportunity Analysis and Industry Forecast, 2020–2027. Available online: https://www.alliedmarketresearch.com/surfactant-market (accessed on 12 November 2021).
- Tadros, T.F. An Introduction of Surfactants; De Gruyter Graduate: Berlin, Germany, 2014.
- Scorzza, C.; Nieves, J.; Vejar, F.; Bullón, J. Synthesis and Physicochemical Characterization of Anionic Surfactants Derived from Cashew Nut Shell Oil. J. Surfactants Deterg. 2010, 13, 27.
- Koteich Khatib, S.; Bullón, J.; Vivas, J.; Bahsas, A.; Rosales-Oballos, Y.; Marquez, R.; Forgiarini, A.; Salager, J.L. Synthesis, Characterization, Evaluation of Interfacial Properties and Antibacterial Activities of Dicarboxylate Anacardic Acid Derivatives from Cashew Nut Shell Liquid of Anacardium occidentale L. J. Surfactants Deterg. 2020, 23, 503–512.
- Wang, Z.; Yao, S.; Song, K.; Gong, X.; Zhang, S.; Gao, S.; Lu, Z. A bio-based benzoxazine surfactant from amino acids. Green Chem. 2020, 22, 3481–3488.
- Peungjitton, P.; Sangvanich, P.; Pornpakakul, S.; Petsom, A.; Roengsumran, S. Sodium Cardanol Sulfonate Surfactant from Cashew Nut Shell Liquid. J. Surfactants Deterg. 2009, 12, 85–89.
- Ahire, M.B.; Bhagwat, S.S. Novel Ester-linked Anionic Gemini Surfactant: Synthesis, Surface-Active Properties and Antimicrobial Study. J. Surfactants Deterg. 2017, 20, 789–797.
- Wang, J.; Wang, Y.W.; Li, C.Q.; Li, J. Synthesis and Surface Activity of Biomass Cardanol Sulfonate Surfactant. Adv. Mater. Res. 2011, 183–185, 1534–1538.
- Castro Dantas, T.N.; Vale, T.Y.F.; Dantas Neto, A.A.; Scatena, H., Jr.; Moura, M.C.P.A. Micellization study and adsorption properties of an ionic surfactant synthesized from hydrogenated cardanol in air–water and in air–brine interfaces. Colloid Polym. Sci. 2009, 287, 81–87.
- Li, M.; Quan, C. Novel Sodium Salt Cardanol Surfactant and Preparation Method Thereof; Shanghai Meidong Biological Materials Co. Ltd.: Shanghai, China, 2009.
- Li, M.; Quan, C. Novel Calcium Salt Cardanol Surfactant and Preparation Method Thereof; Shanghai Meidong Biological Materials Co. Ltd.: Shanghai, China, 2009.
- Li, M.; Quan, C. Novel Sylvite Cardanol Surfactant and Preparation Method Thereof; Shanghai Meidong Biological Materials Co. Ltd.: Shanghai, China, 2009.
- Kattimuttathu, I.S.; Foerst, G.; Schubert, R.; Bartsch, E. Synthesis and Micellization Properties of New Anionic Reactive Surfactants Based on Hydrogenated Cardanol. J. Surfactants Deterg. 2012, 15, 207–215.
- Jin, Z.; Zhao, S.; Xu, Z.; Gong, Q.; Zhang, L.; Zhang, L.; Luo, L. Saturated Anacardol Ether Sulfonate Surfactant as Well as Preparation Method and Application Thereof; Technical Institute of Physics and Chemistry of CAS: Beijing, China, 2014.
- Zhou, Y.; Su, P.; Xu, B.; Han, F. A Kind of Anacardol Dimeric Surfactant and Preparation Method Thereof. CN Patent CN103725279A, 23 January 2014.
- Gulati, A.S.; Krishnamachar, V.C.; Rao, B.C.S. Quaternary nitrogen germicides derived from the monophenolic components of cashew nut shell liquid. Rao Indian J. Chem 1964, 2, 114.
- Ezzat, A.O.; Atta, A.M.; Al-Lohedan, H.A.; Abdullah, M.M.S.; Hashem, A.I. Synthesis and Application of Poly(ionic liquid) Based on Cardanol as Demulsifier for Heavy Crude Oil Water Emulsions. Energy Fuels 2018, 32, 214–225.
- Luo, Y.; Liang, W.; Ma, W.; Wang, P.; Zhu, T.; Xue, S.; Yuan, Z.; Gao, H.; Chen, Y.; Wang, Y. Cardanol-derived cationic surfactants enabling the superior antibacterial activity of single-walled carbon nanotubes. Nanotechnology 2020, 31, 265603.
- Sunnapu, O.; Ravipati, P.; Srinath, P.; Kalita, S.; Bhat, P.P.; Harshitha, S.R.; Sekar, K.; Vemula, P.K.; Mahato, M. Design of cationic amphiphiles for generating self-assembled soft nanostructures, micelles and hydrogels. Bull. Mater. Sci. 2020, 43, 172.
- Mondal, M.H.; Malik, S.; Roy, A.; Saha, R.; Saha, B. Modernization of surfactant chemistry in the age of gemini and bio-surfactants: A review. RSC Adv. 2015, 5, 92707–92718.
- Zana, R. Gemini (dimeric) surfactants. Curr. Opin. Colloid Interface Sci. 1996, 1, 566–571.
