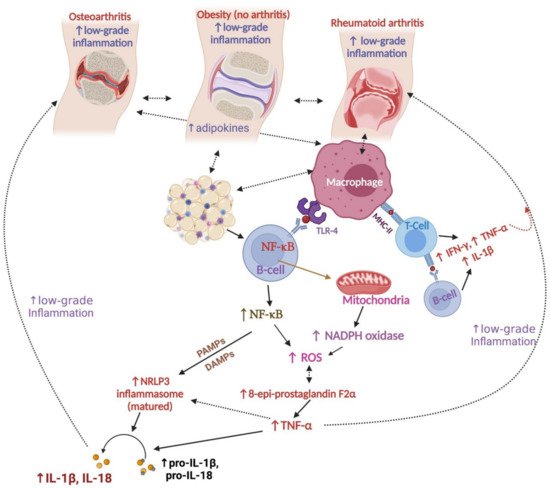
Obesity is a significant health concern, as it causes a massive cascade of chronic inflammations and multiple morbidities. Rheumatoid arthritis and osteoarthritis are chronic inflammatory conditions and often manifest as comorbidities of obesity. Adipose tissues serve as a reservoir of energy as well as releasing several inflammatory cytokines (including IL-6, IFN-γ, and TNF-α) that stimulate low-grade chronic inflammatory conditions such as rheumatoid arthritis, osteoarthritis, diabetes, hypertension, cardiovascular disorders, fatty liver disease, oxidative stress, and chronic kidney diseases. Dietary intake, low physical activity, unhealthy lifestyle, smoking, alcohol consumption, and genetic and environmental factors can influence obesity and arthritis. Current arthritis management using modern medicines produces various adverse reactions. Medicinal plants have been a significant part of traditional medicine, and various plants and phytochemicals have shown effectiveness against arthritis and obesity; however, scientifically, this traditional plant-based treatment option needs validation through proper clinical trials and toxicity tests. In addition, essential oils obtained from aromatic plants are being widely used for complementary therapy (e.g., aromatherapy, smelling, spicing, and consumption with food) against arthritis and obesity; scientific evidence is necessary to support their effectiveness.
- rheumatoid arthritis
- obesity
- spice
- medicinal plant
- aromatic plant
- essential oil
- osteoarthritis
- comorbidity
1. Introduction
1.1. Obesity and Inflammation
1.2. Influence of Dietary Habits during Childhood on Obesity and Inflammation
2. Arthritis and Inflammation
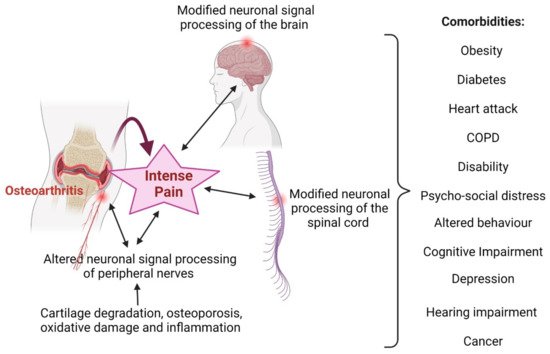
2.1. Osteoarthritis and Inflammation
2.2. Brief Pathophysiology of Rheumatoid Arthritis (RA)
2.2.1. RA and Inflammation
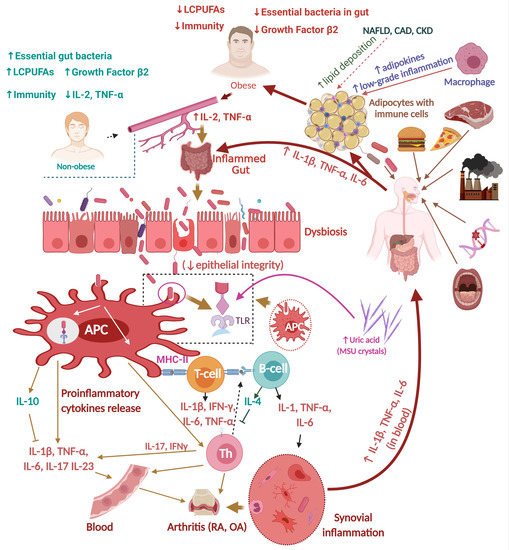
2.2.2. RA, Gut Dysbiosis, and Inflammation
3. Relationships between Obesity and Arthritis
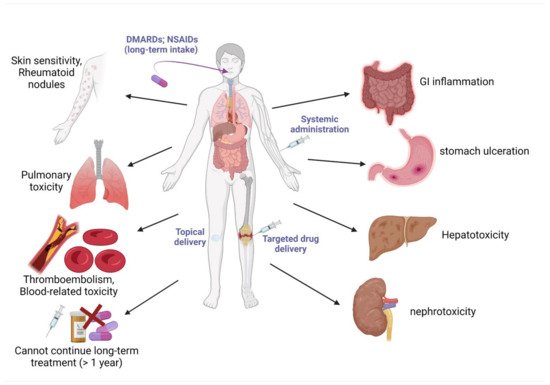
4. Current Drugs for the Management of Obesity and Arthritis
This entry is adapted from the peer-reviewed paper 10.3390/nu14050985
References
- World Health Organization. Obesity and Overweight. 9 June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 August 2021).
- Kortt, M.; Baldry, J. The association between musculoskeletal disorders and obesity. Aust. Health Rev. 2002, 25, 207–214.
- Poirier, P.; Després, J.-P. Obésité et maladies cardiovasculaires. M/S Méd. Sci. 2003, 19, 943–949.
- Parmar, M.Y. Obesity and type 2 diabetes mellitus. Integr. Obes. Diabetes 2018, 4, 1–2.
- Basen-Engquist, K.; Chang, M. Obesity and cancer risk: Recent review and evidence. Curr. Oncol. Rep. 2010, 13, 71–76.
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017, 376, 254–266.
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013, 17, 644–656.
- Redinger, R.N. The pathophysiology of obesity and its clinical manifestations. Gastroenterol. Hepatol. 2007, 3, 856–863.
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 2015, 10, e0121971.
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355.
- Lee, H.; Lee, I.S.; Choue, R. Obesity, inflammation and diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152.
- Knebel, B.; Fahlbusch, P.; Poschmann, G.; Dille, M.; Wahlers, N.; Stühler, K.; Hartwig, S.; Lehr, S.; Schiller, M.; Jacob, S.; et al. Adipokinome signatures in obese mouse models reflect adipose tissue health and are associated with serum lipid composition. Int. J. Mol. Sci. 2019, 20, 2559.
- Eissing, L.; Scherer, T.; Tödter, K.; Knippschild, U.; Greve, J.W.; Buurman, W.A.; Pinnschmidt, H.O.; Rensen, S.S.; Wolf, A.M.; Bartelt, A.; et al. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat. Commun. 2013, 4, 1528.
- Adolph, T.E.; Grander, C.; Grabherr, F.; Tilg, H. Adipokines and non-alcoholic fatty liver disease: Multiple interactions. Int. J. Mol. Sci. 2017, 18, 1649.
- Frühbeck, G.; Gomez-Ambrosi, J.; Muruzabal, F.J.; Burrell, M. The adipocyte: A model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am. J. Physiol. Metab. 2001, 280, E827–E847.
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339.
- Ellulu, M.S.; Patimah, I.; KhazáAi, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863.
- Das, U. Is obesity an inflammatory condition? Nutrition 2001, 17, 953–966.
- Hill, J.O. A new way of looking at obesity. Nutrition 2001, 17, 975–976.
- Vozarova, B.; Weyer, C.; Hanson, K.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes. Res. 2001, 9, 414–417.
- Esposito, K.; Pontillo, A.; Ciotola, M.; Di Palo, C.; Grella, E.; Nicoletti, G.; Giugliano, D. Weight loss reduces interleukin-18 levels in obese women. J. Clin. Endocrinol. Metab. 2002, 87, 3864–3866.
- Rodríguez-Hernández, H.; Simental-Mendía, L.E.; Rodríguez-Ramírez, G.; Reyes-Romero, M.A. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. Int. J. Endocrinol. 2013, 2013, 678159.
- Palmeira, P.; Carneiro-Sampaio, M. Immunology of breast milk. Rev. Assoc. Méd. Bras. 2016, 62, 584–593.
- Burris, A.D.; Pizzarello, C.; Järvinen, K.M. Immunologic components in human milk and allergic diseases with focus on food allergy. Semin. Perinatol. 2020, 45, 151386.
- Dain, A.; Repossi, G.; Diaz-Gerevini, G.T.; Vanamala, J.; Das, U.N.; Eynard, A.R. Long chain polyunsaturated fatty acids (LCPUFAs) and nordihydroguaiaretic acid (NDGA) modulate metabolic and inflammatory markers in a spontaneous type 2 diabetes mellitus model (Stillman Salgado rats). Lipids Health Dis. 2016, 15, 205.
- Das, U.N. Long-chain polyunsaturated fatty acids and diabetes mellitus. Am. J. Clin. Nutr. 2002, 75, 780–781.
- Hanson, L.; Korotkova, M. The role of breastfeeding in prevention of neonatal infection. Semin. Neonatol. 2002, 7, 275–281.
- Quinello, C.; Quintilio, W.; Carneiro-Sampaio, M.; Palmeira, P. Passive Acquisition of protective antibodies reactive with Bordetella pertussis in newborns via placental transfer and breast-feeding. Scand. J. Immunol. 2010, 72, 66–73.
- Kainonen, E.; Rautava, S.; Isolauri, E. Immunological programming by breast milk creates an anti-inflammatory cytokine milieu in breast-fed infants compared to formula-fed infants. Br. J. Nutr. 2012, 109, 1962–1970.
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A reappraisal. Mech. Ageing Dev. 2019, 178, 46–63.
- Xie, C.; Chen, Q. Adipokines: New therapeutic target for osteoarthritis? Curr. Rheumatol. Rep. 2019, 21, 71.
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400.
- Senthelal, S.; Li, J.; Goyal, A.; Bansal, P.; Thomas, M.A. Arthritis. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Wallace, J.L. Polypharmacy of osteoarthritis: The perfect intestinal storm. Am. J. Dig. Dis. 2013, 58, 3088–3093.
- Xia, B.; Chen, D.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Res. 2014, 95, 495–505.
- So, A. Developments in the scientific and clinical understanding of gout. Arthritis Res. Ther. 2008, 10, 221.
- Goo, B.; Lee, J.; Park, C.; Yune, T.; Park, Y. Bee venom alleviated edema and pain in monosodium urate crystals-induced gouty arthritis in rat by inhibiting inflammation. Toxins 2021, 13, 661.
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350.
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447.
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241.
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687.
- Millerand, M.; Berenbaum, F.; Jacques, C. Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 48–56.
- Zhao, L.; Xing, R.; Wang, P.; Zhang, N.; Yin, S.; Li, X.; Zhang, L. NLRP1 and NLRP3 inflammasomes mediate LPS/ATP-induced pyroptosis in knee osteoarthritis. Mol. Med. Rep. 2018, 17, 5463–5469.
- Denoble, A.E.; Huffman, K.M.; Stabler, T.V.; Kelly, S.J.; Hershfield, M.S.; McDaniel, G.E.; Coleman, R.E.; Kraus, V.B. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc. Natl. Acad. Sci. USA 2011, 108, 2088–2093.
- Fiddis, R.W.; Vlachos, N.; Calvert, P.D. Studies of urate crystallisation in relation to gout. Ann. Rheum. Dis. 1983, 42 (Suppl. 1), 12–15.
- Wilson, L.; Saseen, J.J. Gouty Arthritis: A review of acute management and prevention. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 906–922.
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castaneda, J.; Coyfish, M.; Guillo, S.; Jansen, T.; Janssens, H.; et al. 2018 updated European league against rheumatism evidence-based recommendations for the diagnosis of gout. Ann. Rheum. Dis. 2019, 79, 31–38.
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94.
- Reginato, A.M.; Olsen, B.R. The role of structural genes in the pathogenesis of osteoarthritic disorders. Arthritis Res. Ther. 2002, 4, 337–345.
- Held, F.P.; Blyth, F.; Gnjidic, D.; Hirani, V.; Naganathan, V.; Waite, L.M.; Seibel, M.J.; Rollo, J.; Handelsman, D.J.; Cumming, R.G.; et al. Association rules analysis of comorbidity and multimorbidity: The concord health and aging in men project. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 625–631.
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759.
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50.
- Kulkarni, K.; Karssiens, T.; Kumar, V.; Pandit, H. Obesity and osteoarthritis. Maturitas 2016, 89, 22–28.
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37, 3–6.
- Wang, Z.; Singh, A.; Jones, G.; Winzenberg, T.; Ding, C.; Chopra, A.; Das, S.; Danda, D.; Laslett, L.; Antony, B. Efficacy and safety of turmeric extracts for the treatment of knee osteoarthritis: A systematic review and meta-analysis of randomised controlled trials. Curr. Rheumatol. Rep. 2021, 23, 11.
- Scarpignato, C.; Hunt, R.H. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: Clinical picture, pathogenesis, and prevention. Gastroenterol. Clin. N. Am. 2010, 39, 433–464.
- Scarpignato, C. Piroxicam-β-cyclodextrin: A GI safer piroxicam. Curr. Med. Chem. 2013, 20, 2415–2437.
- Wehling, M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: Management and mitigation of risks and adverse effects. Eur. J. Clin. Pharmacol. 2014, 70, 1159–1172.
- Van Laar, M.; Pergolizzi, J.V., Jr.; Mellinghoff, H.-U.; Merchante, I.M.; Nalamachu, S.; O’Brien, J.; Perrot, S.; Raffa, R.B. Pain treatment in arthritis-related pain: Beyond NSAIDs. Open Rheumatol. J. 2012, 6, 320–330.
- Courtney, P. Key questions concerning paracetamol and NSAIDs for osteoarthritis. Ann. Rheum. Dis. 2002, 61, 767–773.
- Pelletier, J.-P.; Martel-Pelletier, J.; Rannou, F.; Cooper, C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2015, 45, S22–S27.
- Da Costa, B.R.; Reichenbach, S.; Keller, N.; Nartey, L.; Wandel, S.; Jüni, P.; Trelle, S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017, 390, e21–e33.
- Solomon, D.H.; Husni, M.E.; Mph, K.E.W.; Rn, L.M.W.; Borer, J.S.; Graham, D.Y.; Libby, P.; Lincoff, A.M.; Lüscher, T.F.; Menon, V.; et al. Differences in safety of nonsteroidal antiinflammatory drugs in patients with osteoarthritis and patients with rheumatoid arthritis. Arthritis Rheumatol. 2017, 70, 537–546.
- Paul, A.K.; Gueven, N.; Dietis, N. Morphine dosing strategy plays a key role in the generation and duration of the produced antinociceptive tolerance. Neuropharmacology 2017, 121, 158–166.
- Malfait, A.-M.; Schnitzer, T.J. Towards a mechanism-based approach to pain management in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 654–664.
- Ragni, E.; Mangiavini, L.; Viganò, M.; Brini, A.T.; Peretti, G.M.; Banfi, G.; De Girolamo, L. Management of osteoarthritis during the COVID-19 pandemic. Clin. Pharmacol. Ther. 2020, 108, 719–729.
- The Royal Australian College of General Practitioners. Guideline for the Management of Knee and Hip Osteoarthritis, 2nd ed.; The Royal Australian College of General Practitioners: East Melbourne, Australia, 2018.
- Alamanda, V.K.; Wally, M.K.; Seymour, R.B.; Springer, B.D.; Hsu, J.R.; Beuhler, M.; Bosse, M.J.; Gibbs, M.; Griggs, C.; Jarrett, S.; et al. Prevalence of opioid and benzodiazepine prescriptions for osteoarthritis. Arthritis Care Res. 2019, 72, 1081–1086.
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid analgesia and opioid-induced adverse effects: A review. Pharmaceuticals 2021, 14, 1091.
- Paul, A.K.; Lewis, R.J. Pain management in older adults: Facts to consider. Pain 2022, 163, e497–e498.
- Mushtaq, S.; Choudhary, R.; Scanzello, C.R. Non-surgical treatment of osteoarthritis-related pain in the elderly. Curr. Rev. Musculoskelet. Med. 2011, 4, 113–122.
- Serhal, L.; Lwin, M.N.; Holroyd, C.; Edwards, C.J. Rheumatoid arthritis in the elderly: Characteristics and treatment considerations. Autoimmun. Rev. 2020, 19, 102528.
- Xu, L.; Feng, X.; Tan, W.; Gu, W.; Guo, D.; Zhang, M.; Wang, F. IL-29 enhances Toll-like receptor-mediated IL-6 and IL-8 production by the synovial fibroblasts from rheumatoid arthritis patients. Arthritis Res. Ther. 2013, 15, R170.
- Thompson, C.; Davies, R.; Choy, E. Anti cytokine therapy in chronic inflammatory arthritis. Cytokine 2016, 86, 92–99.
- Kishimoto, T. Discovery of IL-6 and Development of anti-IL-6R antibody. Keio J. Med. 2019, 68, 96.
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171.
- Van Hamburg, J.P.; Tas, S.W. Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J. Autoimmun. 2018, 87, 69–81.
- Alam, J.; Jantan, I.; Bukhari, S.N.A. Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed. Pharmacother. 2017, 92, 615–633.
- Chen, J.-Q.; Szodoray, P.; Zeher, M. Toll-like receptor pathways in autoimmune diseases. Clin. Rev. Allergy Immunol. 2015, 50, 1–17.
- McGarry, T.; Veale, D.J.; Gao, W.; Orr, C.; Fearon, U.; Connolly, M. Toll-like receptor 2 (TLR2) induces migration and invasive mechanisms in rheumatoid arthritis. Arthritis Res. Ther. 2015, 17, 153.
- Piccinini, A.M.; Williams, L.; McCann, F.E.; Midwood, K.S. Investigating the role of toll-like receptors in models of arthritis. In Toll-Like Receptors; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1390, pp. 351–381.
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219.
- Malemud, C.J. Matrix metalloproteinases and synovial joint pathology. Prog. Mol. Biol. Transl. Sci. 2017, 148, 305–325.
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Harris, H.E.; Ulfgren, A.K.; Rantapää-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46.
- Möller, B.; Kollert, F.; Sculean, A.; Villiger, P.M. Infectious triggers in periodontitis and the gut in rheumatoid arthritis (RA): A complex story about association and causality. Front. Immunol. 2020, 11, 1108.
- Favalli, E.G.; Biggioggero, M.; Crotti, C.; Becciolini, A.; Raimondo, M.G.; Meroni, P.L. Sex and management of rheumatoid arthritis. Clin. Rev. Allergy Immunol. 2018, 56, 333–345.
- Islander, U.; Jochems, C.; Lagerquist, M.K.; Forsblad-D’Elia, H.; Carlsten, H. Estrogens in rheumatoid arthritis; the immune system and bone. Mol. Cell. Endocrinol. 2011, 335, 14–29.
- Fert-Bober, J.; Darrah, E.; Andrade, F. Insights into the study and origin of the citrullinome in rheumatoid arthritis. Immunol. Rev. 2019, 294, 133–147.
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648.
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43.
- He, X.; Zhao, S.; Li, Y. Faecalibacterium prausnitzii: A next-generation probiotic in gut disease improvement. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6666114.
- Chu, X.-J.; Cao, N.-W.; Zhou, H.-Y.; Meng, X.; Guo, B.; Zhang, H.-Y.; Li, B.-Z. The oral and gut microbiome in rheumatoid arthritis patients: A systematic review. Rheumatology 2020, 60, 1054–1066.
- Tanaka, S.; Yoshida, M.; Murakami, Y.; Ogiwara, T.; Shoji, M.; Kobayashi, S.; Watanabe, S.; Machino, M.; Fujisawa, S. The Relationship of Prevotella intermedia, Prevotella nigrescens and Prevotella melaninogenica in the supragingival plaque of children, caries and oral malodor. J. Clin. Pediatr. Dent. 2008, 32, 195–200.
- Ceccarelli, F.; Saccucci, M.; Di Carlo, G.; Lucchetti, R.; Pilloni, A.; Pranno, N.; Luzzi, V.; Valesini, G.; Polimeni, A. Periodontitis and rheumatoid arthritis: The same inflammatory mediators? Mediat. Inflamm. 2019, 2019, 6034546.
- Paul, A.K.; Paul, A.; Jahan, R.; Jannat, K.; Bondhon, T.A.; Hasan, A.; Nissapatorn, V.; Pereira, M.L.; Wilairatana, P.; Rahmatullah, M. Probiotics and amelioration of rheumatoid arthritis: Significant roles of Lactobacillus casei and Lactobacillus acidophilus. Microorganisms 2021, 9, 1070.
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 2016, 68, 2646–2661.
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374.
- Guan, S.-M.; Fu, S.-M.; He, J.-J.; Zhang, M. Prevotella intermedia induces prostaglandin E2 via multiple signaling pathways. J. Dent. Res. 2010, 90, 121–127.
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073.
- Drago, L.; Zuccotti, G.V.; Romanò, C.L.; Goswami, K.; Villafañe, J.H.; Mattina, R.; Parvizi, J. Oral–gut microbiota and arthritis: Is there an evidence-based axis? J. Clin. Med. 2019, 8, 1753.
- Jung, H.; Jung, S.M.; Rim, Y.A.; Park, N.; Nam, Y.; Lee, J.; Park, S.-H.; Ju, J.H. Arthritic role of Porphyromonas gingivalis in collagen-induced arthritis mice. PLoS ONE 2017, 12, e0188698.
- Carrion, J.; Scisci, E.; Miles, B.; Sabino, G.J.; Zeituni, A.E.; Gu, Y.; Bear, A.; Genco, C.A.; Brown, D.L.; Cutler, C.W. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J. Immunol. 2012, 189, 3178–3187.
- Salaffi, F.; Farah, S.; Di Carlo, M. Frailty syndrome in rheumatoid arthritis and symptomatic osteoarthritis: An emerging concept in rheumatology. Acta Bio-Med. Atenei Parm. 2020, 91, 274–296.
- Han, T.; Tajar, A.; Lean, M.E.J. Obesity and weight management in the elderly. Br. Med Bull. 2011, 97, 169–196.
- Han, T.; Wu, F.; Lean, M. Obesity and weight management in the elderly: A focus on men. Best Pr. Res. Clin. Endocrinol. Metab. 2013, 27, 509–525.
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-α and obesity. TNF Pathophysiol. 2010, 11, 145–156.
- Paul, A.K.; Hossain, K.; Mahboob, T.; Nissapatorn, V.; Wilairatana, P.; Jahan, R.; Jannat, K.; Bondhon, T.A.; Hasan, A.; Pereira, M.D.L.; et al. Does oxidative stress management help alleviation of COVID-19 symptoms in patients experiencing diabetes? Nutrients 2022, 14, 321.
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188.
- Ding, S.; Xu, S.; Ma, Y.; Liu, G.; Jang, H.; Fang, J. Modulatory mechanisms of the NLRP3 inflammasomes in diabetes. Biomolecules 2019, 9, 850.
- Liu, Y.; Hazlewood, G.; Kaplan, G.G.; Eksteen, B.; Barnabe, C. Impact of obesity on remission and disease activity in rheumatoid arthritis: A systematic review and meta-analysis. Arthritis Care Res. 2016, 69, 157–165.
- Moroni, L.; Farina, N.; Dagna, L. Obesity and its role in the management of rheumatoid and psoriatic arthritis. Clin. Rheumatol. 2020, 39, 1039–1047.
- Dar, L.; Tiosano, S.; Watad, A.; Bragazzi, N.L.; Zisman, D.; Comaneshter, D.; Cohen, A.; Amital, H. Are obesity and rheumatoid arthritis interrelated? Int. J. Clin. Pr. 2017, 72, e13045.
- King, L.K.; March, L.; Anandacoomarasamy, A. Obesity & osteoarthritis. Indian J. Med. Res. 2013, 138, 185–193.
- Coggon, D.; Reading, I.; Croft, P.; McLaren, M.; Barrett, D.; Cooper, C. Knee osteoarthritis and obesity. Int. J. Obes. 2001, 25, 622–627.
- Kaeley, G.S.; MacCarter, D.K.; Pangan, A.L.; Wang, X.; Kalabic, J.; Ranganath, V.K. Clinical Responses and synovial vascularity in obese rheumatoid arthritis patients treated with adalimumab and methotrexate. J. Rheumatol. 2018, 45, 1628–1635.
- Stebbings, S.; Treharne, G.J. Fatigue in rheumatic disease: An overview. Int. J. Clin. Rheumatol. 2010, 5, 487.
- Vlietstra, L.; Stebbings, S.; Meredith-Jones, K.; Abbott, J.H.; Treharne, G.; Waters, D.L. Sarcopenia in osteoarthritis and rheumatoid arthritis: The association with self-reported fatigue, physical function and obesity. PLoS ONE 2019, 14, e0217462.
- Tournadre, A.; Pereira, B.; Gossec, L.; Soubrier, M.; Dougados, M. Impact of comorbidities on fatigue in rheumatoid arthritis patients: Results from a nurse-led program for comorbidities management (COMEDRA). Jt. Bone Spine 2018, 86, 55–60.
- Van Beers-Tas, M.H.; Turk, S.A.; Van Schaardenburg, D. How does established rheumatoid arthritis develop, and are there possibilities for prevention? Best Pr. Res. Clin. Rheumatol. 2015, 29, 527–542.
- Engelhart, M.; Kondrup, J.; Høie, L.H.; Andersen, V.; Kristensen, J.H.; Heitmann, B.L. Weight reduction in obese patients with rheumatoid arthritis, with preservation of body cell mass and improvement of physical fitness. Clin. Exp. Rheumatol. 1996, 14, 289–293.
- Gudbergsen, H.; Boesen, M.; Lohmander, S.; Christensen, R.; Henriksen, M.; Bartels, E.; Rindel, L.; Aaboe, J.; Danneskiold-Samsøe, B.; Riecke, B.; et al. Weight loss is effective for symptomatic relief in obese subjects with knee osteoarthritis independently of joint damage severity assessed by high-field MRI and radiography. Osteoarthr. Cartil. 2012, 20, 495–502.
- NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Prescription Medications to Treat Overweight & Obesity. Available online: https://www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity (accessed on 15 August 2021).
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European guidelines for obesity management in adults. Obes. Facts 2015, 8, 402–424.
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions. Drug Saf. 2008, 31, 53–65.
- Lei, X.; Ruan, J.; Lai, C.; Sun, Z.; Yang, X. Efficacy and safety of phentermine/topiramate in adults with overweight or obesity: A systematic review and meta-analysis. Obesity 2021, 29, 985–994.
- Siebenhofer, A.; Winterholer, S.; Jeitler, K.; Horvath, K.; Berghold, A.; Krenn, C.; Semlitsch, T. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst. Rev. 2021, 2021, CD007654.
- Tak, Y.J.; Lee, S.Y. Long-term efficacy and safety of anti-obesity treatment: Where do we stand? Curr. Obes. Rep. 2021, 10, 14–30.
- Di Nicolantonio, J.J.; Chatterjee, S.; O’Keefe, J.H.; Meier, P. Lorcaserin for the treatment of obesity? A closer look at its side effects. Open Heart 2014, 1, e000173.
- Paul, A.; Gueven, N.; Dietis, N. Profiling the effects of repetitive morphine administration on motor behavior in rats. Molecules 2021, 26, 4355.
- Paul, A.K.; Gueven, N.; Dietis, N. Data on prolonged morphine-induced antinociception and behavioral inhibition in older rats. Data Brief 2018, 19, 183–188.
- Paul, A.K.; Gueven, N.; Dietis, N. Age-dependent antinociception and behavioral inhibition by morphine. Pharmacol. Biochem. Behav. 2018, 168, 8–16.
- Andreasen, P.B.; Hutters, L. Paracetamol (acetaminophen) clearance in patients with cirrhosis of the liver. Acta Med. Scand. 2009, 205, 99–105.
- Ochs, H.R.; Schuppan, U.; Greenblatt, D.J.; Abernethy, D.R. Reduced distribution and clearance of acetaminophen in patients with congestive heart failure. J. Cardiovasc. Pharmacol. 1983, 5, 697–699.
- Verpeut, J.L.; Bello, N.T. Drug safety evaluation of naltrexone/bupropion for the treatment of obesity. Expert Opin. Drug Saf. 2014, 13, 1–11.
- McIntyre, R.S.; Paron, E.; Burrows, M.; Blavignac, J.; Gould, E.; Camacho, F.; Barakat, M. Psychiatric Safety and Weight loss efficacy of naltrexone/bupropion as add-on to antidepressant therapy in patients with obesity or overweight. J. Affect. Disord. 2021, 289, 167–176.
- Onge, E.S.; Miller, S.A.; Motycka, C. Liraglutide (Saxenda®) as a treatment for obesity. Food Nutr. Sci. 2016, 07, 227–235.
- Therapeutic Guidelines Limited. Principles of Nonsteroidal Anti-Inflammatory Drug Use for Musculoskeletal Conditions in Adults. In eTG Complete Melbourne; Therapeutic Guidelines Limited: West Melbourne, Australia. Available online: https://tgldcdp.tg.org.au/index (accessed on 29 December 2020).
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166.
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516.
- Bramming, M.; Becker, U.; Jørgensen, M.B.; Neermark, S.; Bisgaard, T.; Tolstrup, J.S. Bariatric surgery and risk of alcohol use disorder: A register-based cohort study. Int. J. Epidemiol. 2020, 49, 1826–1835.
