Electrochemical biosensors are undoubtedly the most popular among POC devices due to their high sensitivity, simplicity, low cost, and reliability. The development of these electrochemical devices has continued to grow exponentially since Clark designed the first enzymatic glucose biosensor, which was then improved and became the most commercialized healthcare biosensor. Electrochemical biosensors that use smartphones received great attention as they use a friendly semi-automated user interface with minimum extra tailored hardware. They can also be used at home, offering an interesting, cost-effective alternative.
1. Introduction
When one thinks of POC devices, the literature always refers to the development of low-cost health care technology with low-income populations in mind or places with difficult access. However, in recent years, and more so in the present pandemic context, it can be seen that having POC devices everywhere would greatly help the management and care of patients and health personnel.
Different disciplines can converge into POC devices development, such as chemistry, biology, physics, and engineering; their combination gives rise to an interesting variety of sub-disciplines, such as biosensors, biochips, and microfluidics. When microfluidics is combined with biosensors, the possibilities become limitless. The integration of both technologies provides the possibility of miniaturized devices, an important and highly sought feature for the development of POC devices. Rackus and collaborators propose a Venn diagram, an interesting scheme that summarizes this concept [
1]. They show how these sub-disciplines overlap and work together, and they argue, quite appropriately, that the overlapping of these three fields gives rise to point-of-care systems. However, if electrochemistry is changed by optical or other types of biosensors, this combination is an interesting example of how they are combining to form new application areas, which reveals great opportunities to develop POC devices.
Figure 1 show this idea by modifying the first Venn diagram proposed by Rackus et al. It also includes the use of new materials, such as paper-based chips, new fabrication procedures, and different analytical methods.
Figure 1. Generalization of the Venn diagram proposed by Rackus et al. [
1]. It shows the interaction of biosensors, microfluidics, and different technologies and analytical methods, which gives rise to POC devices.
On the other hand, in the last decade, there was an explosion of smartphone-based biosensors [
2,
3,
4]. The ubiquity of smartphones throughout the world has brought about new opportunities to bring POC devices near the patients for portable healthcare monitoring, taking advantage of the characteristics of computing power, network connectivity, battery, and cameras of these devices. This can help both patients and physicians for the faster, more efficient and reliable resolution of any health problem that may arise at home or outside the context of healthcare centers. In addition, the widespread connectivity options of the current wireless telecommunication infrastructure make the smartphone a ubiquitous platform worthy of using in order to develop biosensing and diagnostics platforms, especially for point-of-care and telemedicine applications. POC devices help to bring diagnoses closer to the patient by providing faster and more frequent feedback with the physicians [
5]. The latter is the
raison d’être of smart POC biosensors.
Wearable biosensors are also important to consider but deserve special consideration, so they will only be considered if there are any special cases. Readers who are interested in this particular topic can refer to very complete and excellent reviews in the bibliography such as those of Ray et al. [
6]; Kim et al. (2015 and 2018) [
7,
8]; Ajami and Teimouri [
9]; Bandodkar et al. [
10]; Nag et al. [
11]; Tamsin [
12]; Rodrigues et al. [
13]; Chung et al. [
14]; Lee et al., to name only a few.
Electrochemical biosensors are undoubtedly the most popular among POC devices due to their high sensitivity, simplicity, low cost, and reliability. The development of these electrochemical devices has continued to grow exponentially since Clark designed the first enzymatic glucose biosensor [
15], which was then improved and became the most commercialized healthcare biosensor. In the last five years, electrochemical biosensors that use smartphones received great attention as they use a friendly semi-automated user interface with minimum extra tailored hardware. They can also be used at home, offering an interesting, cost-effective alternative. A very interesting review by Sun and Hall presents a study on the different technologies used in electrochemical smartphone-based biosensors in terms of the voltage sources used, the power required in each case, and the resolution and detection limit characteristics [
5].
Optical biosensors also showed significant growth in recent years, even more so with the use of smartphones that allow their use as transmitters or receivers of optical signals. On the other hand, the introduction of paper as a substrate for the development of analytical systems proved to be the most chosen in recent years. This type of substrate allows for the implementation of both electrochemical and optical biosensors.
Microfluidic paper-based analytical devices (µPAD) applied to biosensing technologies were widely developed since their first proposal by the Whitesides group in 2007 [
16]. Paper possess networks of hydrophilic/hydrophobic micro channels, which make quantitative analysis possible for their potential application in biochemical environments in healthcare. Furthermore, focusing on the point-of-care approach, paper-based sensing devices were connected with optical or colorimetric reactions in order to obtain rapid and on-site results. However, paper also presents some limitations, such as reproducibility and repeatability, and the measurements are more difficult to automate. These limitations impact the quality of the results, mainly regarding naked-eye detections where the operator may have subjective interpretation on different results. In order to tackle these limitations for paper-based optical devices and improve their outcome, in recent years, these devices were combined with smartphone technologies to capture, analyze, and quantify analytical measurements, having a better and more robust performance [
17].
2. Current State of Art and Trends in Smartphone-Based Sensors Field
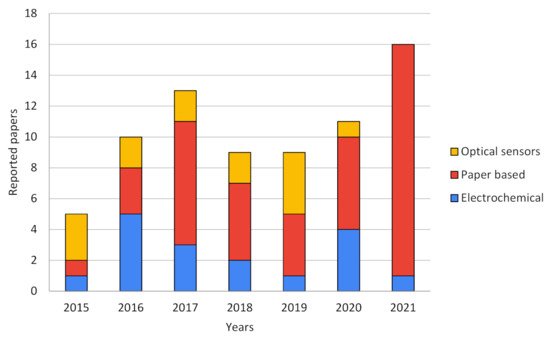
Both scientific research articles and patent databases were consulted in order to elucidate the current state of the art trends in smartphone-based sensors technologies. In order to simplify the classification of each reviewed paper or patent, technologies were segmented into three categories: (A) electrochemical sensors, (B) optical sensors, and (C) paper-based sensors. Figure 2 show the trends in each technology over the years reviewed, expressed in numbers of published papers related to smartphone-based sensors.
Figure 2. Publications trend of smartphone-based biosensors over the years by type of technology.
As can be seen in
Figure 2, in the last five years, the popularity of smartphone-based sensors, measured as the number of publications, increased in general and is doubtless linked to the rapid evolution and development of smartphones due to their processing power and the better performance of their tools such as cameras and light sensors [
18,
19]. It can be seen that electrochemical and optical sensors were featured in most of the publications until approximately 2016, but further and near 2020 and 2021, paper-based sensors mainly occupy the major scene in this field. This evolution trend can be explained due to the type of strategy used by researchers when profiting the smartphone features. Electrochemical sensors use smartphones not only as point-of-care potentiometric devices and signal processing but also as the power source of the whole biosensor. Optical devices generally need specific appliances, hardware, and a complex isolated environment in order to achieve good results. Some of these drawbacks favor paper-based electrochemical proposals due to their cheaper fabrication and simpler setups to achieve comparable results to pure electrochemical devices. As for pure optical sensors, a fairly stable development can be observed over the years reviewed. This could be due to the complexity of the optical systems required, which have apparently been replaced by paper-based optical sensors that take advantage of the advent of better cameras, improved light sensors, and more powerful image processing systems in smartphones [
20].
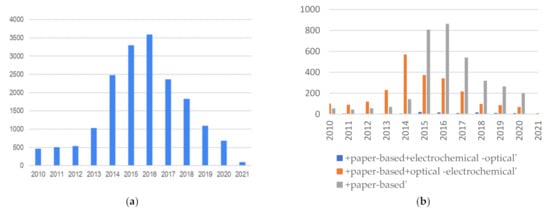
When analyzing overall patent applications by filtering in a wider time window, a very interesting response can be seen regarding patent applications with the search pattern “biosensor + smartphone + point-of-care” or “biosensor + smartphone + poc”. As can be seen in Figure 3, there is a systematic increase in patents from 2010 to around 2016 and then a sharp decrease until 2021. It is interesting to see how smart devices became popular until 2016, but the significant decrease in recent years is striking.
Figure 3. (a) Patent applications “biosensor + smartphone + poc(point-of-care)”; (b) Patent applications for electrochemical, paper-based, and optical smartphone based biosensors.
Figure 3b show the same data but discriminates between the different types of biosensors. The same trend can be observed. The large increase in patent applications in this area in the first half of the decade until 2016 correlates with the advancement of smartphone technology, but it is surprising why, in the second half, they declined rapidly. Perhaps the “Theranos effect” may have played a role that was not minor. On the other hand, frequently, patent applications are carried out with laboratory validations, but later, moving on to the technology implementation stage and application with real samples becomes more difficult. Another fact to consider is that some publications on biosensors are developed on devices that are already widely used, such as those of glucose, for example, and the novelty to make them smart is not enough to achieve a technology replacement by the users. It is expected that the publications of the last years will be delayed since the development of new devices and innovation in the area has grown a lot and, as is known, patents are filed first. Therefore, in this case, the patent applications decreased in recent years.
Another interesting fact is that this effect is not evident for electrochemical sensors. Despite their popularity, they are much less prevalent, but they remain in number throughout the reviewed period.
3. Overview of Reviewed Technologies by Type of Sensors and Commercial Stage
This section presents an overview of the different types of smartphone-based biosensors, taking into account the transduction method and the substrate material. In this way, biosensors were classified as electrochemical, optical, and paper-based biosensors.
3.1. Electrochemical Smartphone-Based Biosensors
The integration of electrochemical POC devices with smartphones is a very promising strategy due to the great improvement of the advantages of each technology. Electrochemical biosensors have high sensitivity and specificity, with the possibility of simple and fast quantitative measurements, all features that can be enhanced with the use of smartphones.
Numerous strategies are used in the development of this type of device, using, for example, smartphones as the electrochemical analyzer or simply to power external dongles. This is an important feature to take into account, that is, the way the measurement module is integrated into the smartphone [
5]. There are wired peripherals, for example, through the USB with OTG (On-The-Go) protocol (a kind of device communication standard), which limits its use depending on the model and brand of the phone or the ones that use the audio headphone port. The wireless peripherals (where the connection is via Bluetooth and near-field communication (NFC)) have the benefits that the measurement electrodes can be integrated near the patient, even being wearable, and the smartphones can be a potential source of energy, signal processing, and are convenient devices for data readout in wearables [
21]. The case of internal dedicated hardware is another method of integration with the smartphone, and although there are some examples of them [
22,
23], the main problem is that developments of this type are made for a particular type of smartphone and therefore are restricted only to that particular type and brand of phone [
5].
Considering the most recent reports on this topic, some examples of electrochemical biosensors that use smartphones are here presented. Table 1 presents the selected publications of the last six years considering the publications that have patents or patent applications, which gives an indication of which technologies would go to the next stage of the application and use in patients. As can be seen, only one-third of the selected publications have application or granted patents.
Table 1. Smartphone-based electrochemical biosensors compared with benchtop techniques.
| Application |
Biosensor Type |
Evaluated in Real Samples? |
Pat. Nº, Year, State |
Improvements of Smart Sensor vs. Benchtop Techniques |
Refs. |
| Secretory leukocyte protease inhibitor (SLPI) but can be applied to different applications |
Immunological |
No. Tested in solutions of different concentrations of the biomarker secretory leukocyte protease inhibitor (SLPI) |
US11166653B2, 2016/2021 [24] |
Electronic module containing a low-power potentiostat that interfaces efficiently with a wide variety of phones through the audio jack to obtain power and communicate. The system uses a microcontroller. Total power consumption: 6.9 mW. Compared with a commercial potentiostat: current from ±300 pA to ±20 µA with a 100 kΩ gain. It can be used to obtain voltammograms. The platform can be used with different brands of smartphones and allows the use of electrochemical biosensors for different applications. |
[25,26] |
| US20210087614A1, 2019 Pending [27] |
| Blood β-ketone (blood β-hydroxybutyrate) |
Enzymatic: β-hydroxybutyrate dehydrogenase method |
Yes. Tested in finger blood |
|
Electrochemical dongle, which is powered by the smartphone through an OTG. It takes chronoamperometric measurements of blood ketone. Linear regression coefficient of 0.987 for a range of 0 to 4 mmol/L of blood β-hydroxybutyrate. The authors were able to demonstrate that the preciseness and stability of the measured data are highly reliable and applicable for clinical use. |
[28] |
| For protein detection: bull serum albumin (BSA) and thrombin |
Immunological for BSA detection and Enzymatic for Thrombin detection |
No. Tested with solutions of different concentrations of BSA and thrombin |
|
Portable transducer and a handheld detector connected via Bluetooth to the smartphone. The detector can perform electrochemical impedance spectroscopy (EIS) (10 Hz to 10 kHz). The system can detect very low concentrations of BSA (1.78 µg/mL) and thrombin (2.97 ng/mL). They related the charge transfer resistance (Rct) with the concentration of BSA or thrombin. The smartphone delivers control commands, receive data signals, and display the Nyquist graph. A designed Android App serves as an interactive interface between the users and the biosensor system. It allows the use of other electrochemical biosensors. |
[29] |
| Glucose concentration |
Enzyme-carbon composite pellets |
No. Tested with solutions of different glucose concentrations |
US20210270766A1, 2018 Pending [30] |
Electrochemical sensor strips consist of carbon electrodes and a second part is composed of the carbon paste GOx biosensor, which can be replaced in each measurement. The biosensor is a compact carbon/GOx/rhodium pill. Measurement compartment: 3D-fabricated smartphone case with a permanently-attached passive sensor strip and a compartment where the biosensor magnetic pellet is placed for each measurement. They developed a portable potentiostat (Texas Instruments CC2541 BLE System on-Chip) communicated wirelessly with the smartphone. Android-based smartphone application developed. |
[31] |
| Alcohol in whole blood samples |
Enzymatic: two enzymes are used, HRP and alcohol oxidase |
Yes. Tested with whole blood |
|
The system combines a three-electrode microfluidic chip with a secondary compact PCB module as a µPotentiostat. Chronoamperometric and CV measurements. Communicated with the smartphone via USB. The novelty of the system is the reusable biosensor concept. Two enzymes, HRP and alcohol oxidase, are immobilized via in situ electrodeposition of a calcium alginate hydrogel for selective ethanol detection. A constant potential of 0 V was applied between WE and Pt RE. The smartphone acts as a simple graphical interface and for cloud connectivity. |
[32] |
| Cancer biomarker microRNAs (miRNAs) |
Genetic: Tris(2-carboxyethyl)phosphine hydrochloride (TCEP)-treated ssDNA probe drop casted onto an rGO/Au composite-modified WE |
No. Tested with miR-21 spiked artificial saliva |
|
The system presents a circuit board as the potentiostat, powered through smartphone On-The-Go (OTG) port and a graphene oxide/gold composite-modified electrode as the biosensor. The circuit board communicates via Bluetooth with the smartphone. A specially designed Android application shows the results. The detection is facilitated via a synthetic ssDNA probe immobilized onto the GO/Au electrode. Good linearity (r2 = 0.99) for the detection of 1×10−4 M to 1×10−12 M of [miR-21]. The sample must be incubated at 40 °C for 1 h for hybridization before electrochemical measurement. |
[33] |
| Reactive oxygen species (ROS) for COVID-19 detection |
MWCNTs on the tip of steel needles of 3 electrodes |
Yes. Tested in Fresh sputum or bronchoalveolar lavage
fluids |
US11181499B2, 2017/2021 [34] |
The system includes a previously patented
electrochemical ROS/H2O2 system consisting of an electrochemical readout board (+/− 0.8 mV, 100 mV s−1, and a
sensing disposable sensor. The group presented an application Patent in 2020 for the electrochemical approach to detect COVID-19, which was granted in 2021. |
[35] |
| US11047824B2, 2020/2021 [36] |
| RNA from SARS-CoV-2 virus |
Genetic: The sequences were provided by the Chinese Center for Disease Control and Prevention (CDC) |
Yes. Tested with extracts from SARS-CoV-2-confirmed patients and recovered patients |
|
It is an ultrasensitive electrochemical biosensor for the detection of the RNA of SARS-CoV-2 by using a smartphone. They used a super sandwich-type recognition strategy without the need for nucleic acid amplification and reverse transcription. For this biosensor, only two copies (10 μL) of SARS-CoV-2 were required per assay to detect a positive sample. Calibrated with concentrations between 10−17–10−12 M, LOD: 3 aM. LOD of the clinical specimen: 200 copies/mL, which was the lowest LOD among the published RNA measurement of SARS-CoV-2 at this moment |
[37] |
The SARS-CoV-2 outbreak, which rapidly evolved into a worldwide pandemic, is an example of a very important event, where smart POC biosensors have become of vital importance to manage the disease and avoid oversaturate health services. Some authors presented interesting mini-reviews of the development of POC biosensors for the detection of COVID-19, where numerous biosensors reported in the bibliography were analyzed and proposed to be perfectly applied in the detection of this new disease with the adequate adaptation of bioreceptors [
38,
39,
40,
41,
42]. In this sense, electrochemical and optical biosensors would be the best suited to implement COVID-19 POC detection [
38]. POC biosensors can provide valuable data for the effective assessment of clinical progress of the symptoms and to provide alertness on the severity or critical trends of infection. Moreover, if these devices are associated with smartphones or direct communication systems with health centers, unnecessary transfers could be avoided, and it would be possible to act more quickly on patients with a poor evolution.
Table 1 reflect two examples of smartphone-based electrochemical biosensors for this application. Reliable biosensors that patients can buy in a pharmacy and make the determination at home will be very useful. Moreover, it seems convenient to develop biosensors to determine other useful parameters that, together with pulse oximetry determinations, avoid the unnecessary transfer of patients to hospitals or health care centers. Examples of these are the biosensor proposed by Miripour et al. for the detection of ROS species [
35] or that of Baraket et al., who already in 2017, proposed a biosensor for the detection of cytokines [
43]. Non-cytokine protein biomarkers such as C-reactive protein and D-dimer (a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis, which is elevated in patients with COVID-19) or other biomarkers that can also be found in whole blood, serum, urine, saliva, or sweat, can also be used as important biomarkers for monitoring the disease at home. The connection of these biosensors to smartphone systems would allow not only remote control by doctors but also the protection of all health personnel and the general population.
Taking into account all applications of electrochemical smartphone-based pure electrochemical biosensors, it can be seen from Table 1 that only one-third of the reviewed papers were found to have patents or related ones. This may be due to many factors, from little practice of patenting in the countries where the works come from to difficulty in the effective transfer of technology to the market, or the lack of clinical importance of the detected analytes from a POC detection point of view.
3.2. Optical Smartphone-Based Biosensors
The following examples illustrate some of the most remarkable proposals regarding this area, presented in the literature between 2015–2020. The use of microscopy in order to achieve optical detection of biosensing and diagnostic devices is the most common strategy since it provides reliable information and on-site results compatible with point-of-care devices. Nevertheless, microscopy devices are high-quality performance equipment that present some inescapable requirements such as proper infrastructure for its size, high qualified operators, and sometimes high-cost supplies. On the other hand, image analysis for the transduction and quantification of radiation emission or color amount cast by analyte recognition demands using dedicated software in order to obtain information from a sample. For several years, most of these informatics tools were only driven on personal computers or specific equipment, but with the explosive development of mobile applications and rapid enhancement of the mobile processors and computing capacity, the analyzing tools are nowadays within easy reach.
In order to keep using the benefits of microscopy techniques, using image analysis tools, and looking forward to the point-of-care approach, these authors used convenient smartphone features to sense and diagnose biological analytes. Table 2 illustrate some of the most remarkable proposals regarding this area, presented in the literature in the mentioned period.
Table 2. Smartphone-based optical biosensors compared with benchtop techniques.
| Application |
Biosensor Type |
Evaluated in Real Samples? |
Pat. Nº, Year, State |
Improvements of Smart Sensor vs. Benchtop Techniques |
Ref. |
| H2O2 , Glucose and Catechol biosensor |
Enzymatic: GOX and tyrosinase over poly(aniline-co-anthranilic acid) |
Yes. Food and pharmaceutical samples |
|
Polymeric substrate material and image processing software provided a great correlation with benchtop techniques and higher LOD. |
[44] |
| HIV and Hepatitis B biosensor |
DNA/RNA-linked biosensor |
Yes. Plasma samples |
WO2014089700A1, 2013 Pending [45] |
They were able to detect between 103 to 109 copies/mL over a 20µL sample and differentiate patients with HIV from those with HBV on the mono-infection assay and multiplexed detection of both of them in a co-infection assay. The results were quite well-correlated compared to benchtop equipment measurements. |
[46] |
| Hemoglobin and HIV biosensor |
Immunosensor |
Yes. Blood samples |
WO2016025698A1, 2014 Pending [47] |
It consists of a combined pure optical assay and an immunoassay at the same time, and in the same device, without a difficult procedure for handling samples and reagents. The results are in good agreement with their commercial equivalents supported by smartphone technologies. |
[48] |
| E. coli and S. typhimurium biosensor |
Immunosensor |
No |
|
For the first time, a device capable of detecting two genetically related bacteria within a single sample drop is reported, with a LOD of 10−2 CFU/mL, in a fairly short time (12 min), and with a good consistency in comparison with the results obtained in laboratory experiments. |
[49] |
| Zika, Dengue, Chikungunya detector |
DNA/RNA-linked biosensor |
No. Tested in artificial blood, urine, and saliva samples |
US20160025630A1, 2014 Pending [50] |
Detection technique that involves quenching of unincorporated amplification signal reporters (QUASR). Distinctively to other reported LAMP detection modalities, QUASR offers very bright signals, reduces the detection of false-positive amplification, and offers the ability to multiplex two or more targets per reaction. These features can highly reduce reagent costs and dilution needs when sample volume is limiting. A personalized smartphone application (app) controls the isothermal heating module and a LED excitation module via Bluetooth. The app processes images through a novel detection algorithm for multiplexed QUASR assay signals with greater accuracy than conventional image analysis software. |
[51] |
| HIV1-p17, hemagglutinin (HA), and dengue virus type I detector |
Bioluminiscent reporter |
Yes. Blood plasma samples |
WO2019038375A1, 2018 Pending [52] |
The design shows to be an attractive analytical platform for point-of-care antibody detection that dispenses with liquid handling steps that are related to the major issues in immunoassays. |
[53] |
| Inflammation and cell viability biosensors |
Bioluminiscent reporters |
No. Simulated proinflammatory and toxic samples. |
US20120045835A1, 2009 Pending [54] |
A limit of detection for tumor necrosis factor (TNFα) of 0.15 ± 0.05 ng/mL was achieved. This proposal promises to be a useful platform to preliminary screen environmental samples or other types of compounds for on-site detection. |
[55] |
| Hemoglobin sensor |
Label-free detection |
No. Simulated samples. |
US8861086B2, 2014 [56] |
It stands out for its compact size, portability, low cost, the efficiency of optical spectroscopy for quantitative measurement, and ease of data collection, management, and computation. |
[57] |
| US20160296118A1, 2015 Pending [58] |
| Bovine immunoglobulinG (IgG) |
Immunosensor |
No. Spiked buffer solution of IgG protein |
US20190025330A1, 2917 Pending [59] |
In addition to the ability to detect immunoglobulins G, the device can be applied to the sensing of other analytes by properly functionalizing the gold film. The results and sensitivity obtained were comparable to commercial SPR instruments, so being a portable SPR system, it makes it an extremely useful device. |
[60] |
| Chloride, sodium, and zinc in sweat |
Fluorescence |
Yes. Sweat |
US20210145352A1, 2018 Pending [61] |
Through an ultrathin, skin-compatible adhesive layer, the device allows sweat to be collected and distributed to different areas with fluorescent reagents. The device makes it possible to quantitatively determine, in a simple and low-cost device, several biomarkers of sweat at the same time. |
[62] |
| Prostatespecific antigen (PSA) |
Fluorescence |
No. Spiked solution with PSA |
US20120141746A1, 2009 Pending [63] |
The device allows, through simple steps, to quantify different concentrations of PSA by means of fluorescence measurement with a smartphone. This sends the data to the cloud for processing and gives a result in about 1 min. It is not a practical device since it needs an objective lens (magnification 40×) to be able to capture the images with the smartphone. |
[64] |
| JP2008128677A, 2006 Pending [65] |
| WO2017141503A1, 2016 Pending [66] |
The great variety of optical biosensors reported in the bibliography saw their possibilities grow with the incorporation of smartphones as reading devices, actuators, image processors, or connections with the cloud. This incorporation made them very promising devices. In the present case, almost 100% of the publications are supported by patents, so most of them are nearer to a commercial prototype. On the other hand, only a few examples of smartphone-based optical biosensors were presented in Table 2 following the mentioned criteria, since most of the reports correspond to paper-based POC devices, which have even more possibilities and will be treated in the following section of this work.
3.3. Paper-Based Biosensors That Uses Smartphones
In recent years, paper has become an alternative for advanced microfluidic devices, being used as a platform for various analytical and bioanalytical techniques. Within the large volume of POC devices for health care that exists in the market, paper-based biosensors are the most chosen by end-users. Qualities such as their price and their robustness have allowed paper-based POC biosensors to distinguish themselves from other biosensors systems. A market analysis performed by “Grand View Research, USA”, evidenced that the participation of said diagnostic devices in 2016 was approximately $2.2 billion, and it was predicted that its participation would reach $8.35 thousand million for the year 2022 [
67]. Together with qualities such as portability, functionalization and modification, lower cost, ease of manufacturing and transportation, profitability, and biodegradability, these devices recently achieved the SAFE status (affordable, sensitive, specific, easy to use, fast and robust, without equipment, deliverable to all end-users) for POC diagnostics in miniaturized environments [
67,
68] .
Depending on the complexities of fluid handling and precision, paper-based biosensors are classified into dipstick, side-flow assay (LFA), and µPAD, the last one being the only one capable of making a quantitative diagnosis. Due to all the aforementioned benefits of paper-based devices and to allow them to make quantitative or semi-quantitative estimates [
69], research was promoted in recent years on their use as POC assisted by smartphones, strips readers, dedicated electronic devices, signal processing modules, etc. In this way, the development of high-quality peripheral-assisted diagnostic devices and the possibility of generating, at a lower cost, authenticated and organized records for future reference are also promoted [
67,
70].
In this sense, the use of smartphones is a leader over other smart devices for paper-based biosensors due to their easy handling, adjustment, and simplicity for end-users. The joint work of major smartphone manufacturers and healthcare giants has resulted in an overwhelming emergence of smartphone-based diagnostic devices for general health and fitness in this area [
67]. In this way, among the paper-based devices that use intelligent technology, there are those that perform determinations by electrochemiluminescence, electrochemistry, and the most popular, optical measurements.
In the search carried out since 2015, almost 40 publications were found that met the search criteria “+smartphone + point-of-care + paper-based + biosensors”. However, despite the great advantages that these devices present, of all the reports reviewed, only one-third of them had patent applications or granted or related patents. The
Table 3 resumes the most recent published papers that deals with paper-based smart devices, according to the type of analyte to be determined (biochemical analysis, immunoassays, and molecular diagnostics to detect DNA and other biomolecules), as was classified in the paper of Xu et al. [
71], and according to the detection method (optical, electrochemical and electrochemiluminescent). The type of biological sample where the measurements are made is also highlighted. The selection of the papers to include in the
Table 3, was made considering only those that have patents, as it was considered that they would be closest to a real field application device.
Table 3. Smart paper-based biosensor devices classified according to the principle and the type of detection.
| Applications |
Biosensor Type |
Evaluated in Real Samples? |
Pat. Nº, Year, State |
Improvements of Smart Sensor vs. Benchtop Techniques |
Ref. |
| µCTX-II in urine |
Immunological |
No. Tested with artificial urine solution (AUS) with the same composition as real urine |
US20180371529A1, 2015 Pending [72] |
Effective smart optical biosensor, highly correlated with benchtop techniques and higher LOD for the use in patients with complications of renal insufficiency and also for the diagnosis and/or prognosis of osteoarthritis. |
[73] |
| Hemoglobin |
Colorimetric |
Yes. Finger-pricked blood |
WO2021019553A1, 2019 Pending [74]
WO2021019552A1, 2020 Pending [75] |
Fast, sensitive, and specific device for the detection of anemia with good correlation with the results of an automated hematology analyzer and on par with other POC test platforms. The results differ from the pathological estimates within the range of 0.5 g/dL for all severely anemic samples and <1.5 g/dL for the rest of the samples. |
[76] |
| Urinary microbial ATP |
Bioluminescent |
No. A urine sample inoculated with E.Coli was used to simulate a urinary tract infection. |
US8642272B2, 2014 [77] |
First device bioluminescent on paper for the detection of low-cost ATP, based on the reaction of Luciferase/D-Luciferina that exploits the smartphone camera as a detector. The ATP sensing paper includes an Innovator Lyophilized “Nano-Lantern” With Reaction Components. The mentioned patent does not correspond to the device but is related to its manufacturing materials. |
[78] |
| Human IgM and IgG |
Immunological |
Yes. Human serum |
US20210382048A1, 2021 Pending [79] |
This paper device has a detection limit of 100 fg/mL demonstrated for the biomarkers of the IgG and IgM protein, which is higher than the one achieved with a traditional Benchtop ELISA test. It is also a much faster method (<5 min), portable, resistant, stable, and low cost, which uses serum without sample preparation and can be easily discarded. |
[80] |
| SARS-CoV-2 |
Genetic: AuNPs capped with highly specific antisense oligonucleotides (ssDNA) |
Yes. Samples collected from Vero cells infected with SARS-CoV-2 virus and clinical samples |
US20210388454A1, 2020 Pending [81] |
This device can successfully and precisely distinguish the positive samples from Covid-19 from negatives, with sensitivity and specificity of almost 100%. It also presents sensing feasibility even for virus genomic mutation events due to the use of AuNPs, covered with highly specific antisense oligonucleotides (SSDNA) that are simultaneously directed to two separate regions of the same SAR-COV-2 N gene |
[82] |
| Cotinine in Urine |
Immunological-Electrochemical |
Yes. Urine samples of smoker and non-smokers patients |
WO2019139537A1, 2019 Pending [83] |
A simple lateral flow competitive immunochromatography was successfully integrated with the AgNP/HRP/AuNP-modified electrode. Immunoreaction can be monitored by either electrochemical measurement or wireless detection. Wireless sensing was realized for cotinine in the range of 100–1000 ng/mL (R2 = 0.96) in PBS medium. For 1:8 diluted urine samples, the device differentiated positive and negative samples and exhibited cotinine discrimination at levels higher than 12 ng/mL. |
[84] |
| IL-6 levels in blood and respiratory samples |
Immunological |
Yes. Human blood and bronchial aspirate samples |
WO2021048087A1, 2019 Pending [85] |
Paper immunosensor interfaced with a smartphone that generates intense colorimetric signals when the sample contains ultralow concentrations of IL-6. The device combines a paper-based signal amplification mechanism with polymer-filled reservoirs for dispensing antibody-decorated nanoparticles and a bespoken app for color quantification. Semi-quantitative measurements of IL-6 can be facilitated in 10 min with a LOD of 1.3 pg mL−1 and a dynamic range of up to 102 pg mL−1 in diluted blood samples. |
[86] |
It is expected that the number of reports on the development of paper-based smart biosensors will increase and will take a stellar role not only in this pandemic but also in many applications for healthcare. However, although the amount of this type of device on the market and within reach of the people is beginning to increase, it is still scarce.
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering9030101