The refrigerated fresh foods tend to quickly deteriorate along its production and marketing, mainly due to the action of psychrotrophic spoilage microorganisms such as pseudomonads. These bacteria cause discolouration, texture loss and unpleasant flavours, with fatal implications for the quality and shelf life of products. Refirgerated fresh dairy products as well as fresh foods are steadly threatened by these microorganisms against which most control strategies are uneffective.
- pseudomonads
- dairy products
- spoilage traits
- biofilm
- quorum sensing
- metabolic pathways
- QS inhibitors
1. Introduction
2. Pseudomonas spp. as Major Cause of Spoilage in Dairy Chain
Spoilage Traits |
Pseudomonas Species |
Dairy Product |
References |
|---|---|---|---|
Proteolysis |
|||
Milk creaming, sediment formation, gelation, bitterness |
P. fluorescens, P. weihenstephanensis, P. proteolytica, Pseudomonas spp. |
UHT milk | [25][26][27] |
| P. panacis | Skimmed milk | [28] | |
| P. azotoformans | Raw milk | [29][30] | |
| P. gessardii | Milk | [26][31] | |
| P. proteolytica | |||
| P. fluorescens | Unpasteurized goat milk | [32] | |
| Pseudomonas spp. | Non-bovine raw milk | [33] | |
Bitterness, Mozzarella skin wrinkling/peeling, cheese softness, sediment formation |
P. lundensis, P gessardii |
Mozzarella cheese, skimmed milk | [31] |
| P. fluorescens | Crescenza cheese | [34] | |
| P. fluorescens, P. fragi |
Mozzarella cheese | [31] | |
| P. fluorescens, P. putida |
Cheddar cheese | [25] | |
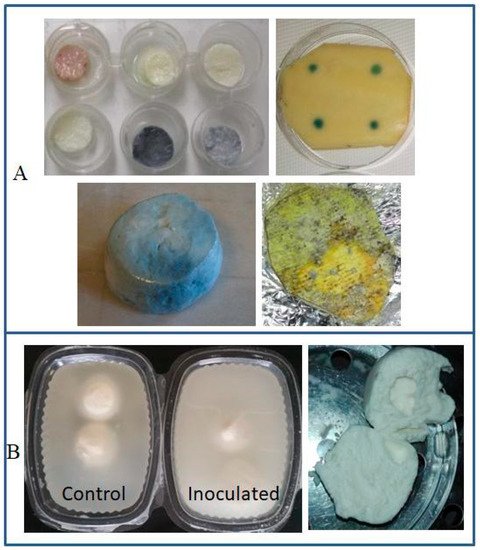
Discoloration |
|||
Blue |
P. fluorescens, P. lactis |
Mozzarella cheese | [8][35] |
| P. fluorescens | Latin-style fresh cheeses | [36][37] | |
| P. carnis, Pseudomonas spp. |
Brazilian fresh soft cheese | [38] | |
| P. fluorescens | Mozzarella processing fluids | [39] | |
Orange orOrange-red-brown |
P. aureofaciens, P. gessardii, P. putida biovar II |
Mozzarella cheese | [40] |
Greenish |
P. fluorescens | ||
Fluorescent(yellow-green) |
P. fluorescens, P. putida, P. palleronii |
||
Grayish |
P. azotoformans | HTST milk | [41] |
| P. fragi | Milk | [40] | |
Black |
P. mephitica, P. nigrifaciens |
Butter | [42] |
Lipolysis |
|||
Rancidityoff-flavorsstraw-berry flavor bitternesssoapy |
Pseudomonas spp., P. fluorescens |
Sterilized milk | [43][44][45] |
| P. fluorescens | Ripened semi-hard cheese | [46][47] | |
| P. fragi, P. putrefaciens, Pseudomonas spp. |
Cream, butter | [44][48][49] | |
| Pseudomonas spp. | Domiati cheese | [50] | |
| Pseudomonas spp. | Soft cheese | [51] | |
Modification of rennet coagulation time and curd firmness |
Pseudomonas spp., P. fluorescens |
Cheese Cheddar cheese |
[44][52] |
2.1. Spoilage Traits Caused by Proteolytic and Lipolytic Activities
2.2. Discoloration as a Spoilage Trait
3. QS-Regulated Spoilage Traits in Dairy-Borne Pseudomonas spp.
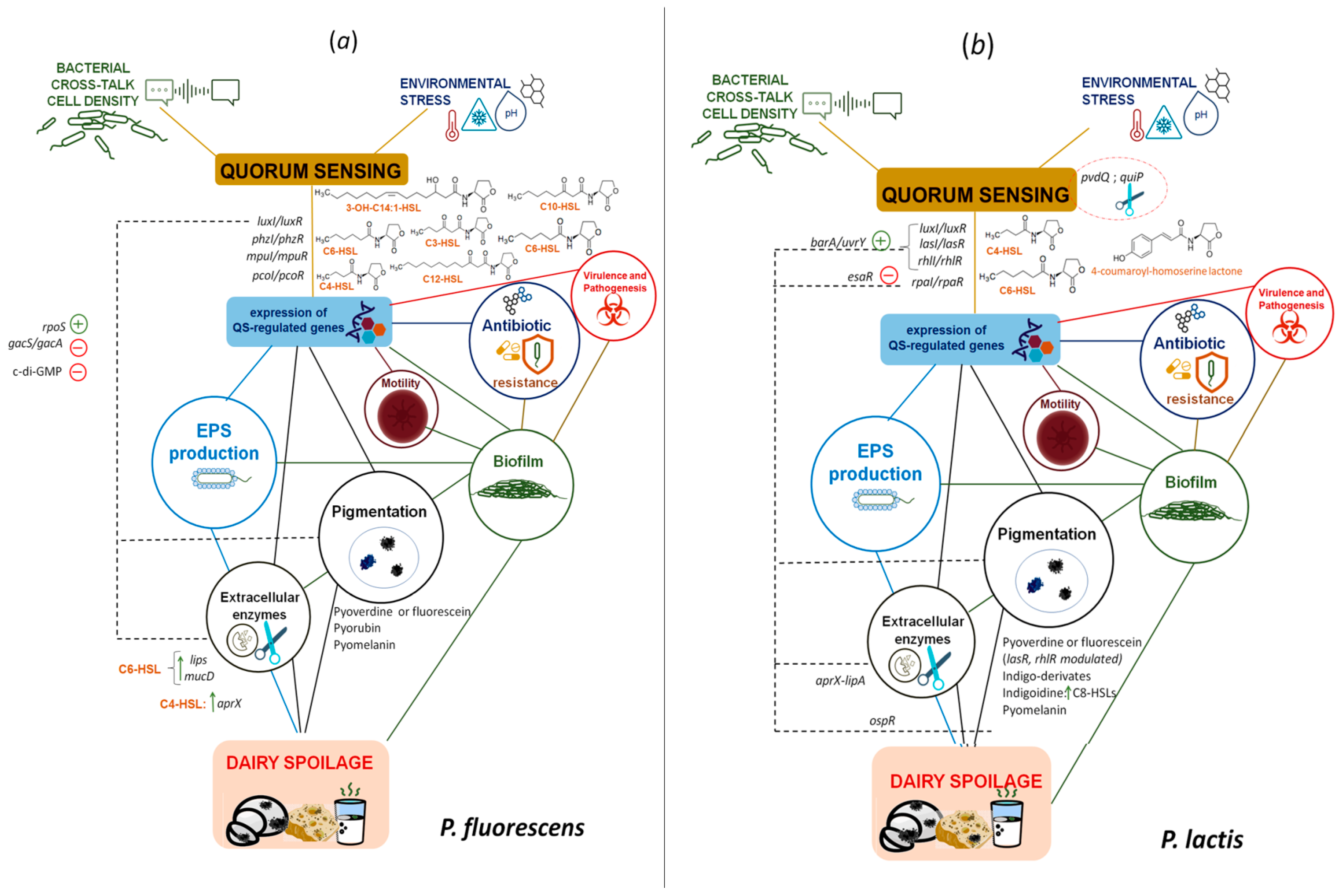
3.1. Proteases, Lipases and Phospholipases
3.2. Pigments
3.3. Off Flavours
4. Conclusion
Food spoilage causes losses of billions of dollars worldwide every year with severe economic and social impacts. In dairy sector new and sustanable strategies are needed to control spoilage of fresh dairy products during their storg at low temperatures. With the evidence of Pseudomonas spp. contamination as a major cause of dairy decay, this entry has deeply investigated all the well-known issues, focusing for the first time on the role of microbial cross-talk in the evolution of spoilage events. Indeed, recently, molecule signals involved in QS have been detected in spoiled products where they affect microbial biodiversity and metabolic activities; these could be exploited as useful markers to monitor the quality of dairy products under storage and prevent spoilage events using natural antimicrobials and QS inhibitors.
This entry is adapted from the peer-reviewed paper 10.3390/foods10123088
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2019. Safeguarding against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019; p. 239.
- Grimaccia, E.; Naccarato, A. Food insecurity in Europe: A gender perspective. Soc. Ind. Res. 2020, 1–19.
- Laborde, D.; Martin, W.; Swinnen, J.; Vos, R. COVID-19 risks to global food security. Science 2020, 369, 500–502.
- UN General Assembly, Transforming Our World: The 2030 Agenda for Sustainable Development, 21 October 2015, A/RES/70/1. Available online: https://www.refworld.org/docid/57b6e3e44 (accessed on 3 November 2021).
- Santeramo, F.G.; Lamonaca, E. Food Loss–Food Waste–Food Security: A New Research Agenda. Sustainability 2021, 13, 4642.
- Martin, N.H.; Boor, K.J.; Wiedmann, M. Effect of post-pasteurization contamination on fluid milk quality. J. Dairy Sci. 2018, 101, 861–870.
- Martin, N.H.; Torres-Frenzel, P.; Wiedmann, M. Controlling dairy product spoilage to reduce food loss and waste. J. Dairy Sci. 2020, 104, 1251–1261.
- Rossi, C.; Serio, A.; Chaves-López, C.; Anniballi, F.; Auricchio, B.; Goffredo, E.; Cenci-Goga, B.T.; Lista, F.; Fillo, S.; Paparella, A. Biofilm formation, pigment production and motility in Pseudomonas spp. isolated from the dairy industry. Food Cont. 2018, 86, 241–248.
- Quintieri, L.; Zühlke, D.; Fanelli, F.; Caputo, L.; Liuzzi, V.C.; Logrieco, A.F.; Hirschfeld, C.; Becher, D.; Riedel, K. Proteomic analysis of the food spoiler Pseudomonas fluorescens ITEM 17298 reveals the antibiofilm activity of the pepsin-digested bovine lactoferrin. Food Microbiol. 2019, 82, 177–193.
- Brown, A.G.; Luke, R.K.J. Siderophore production and utilization by milk spoilage Pseudomonas species. J. Dairy Sci. 2010, 93, 1355–1363.
- Jamuna, B.A.; Ravishankar, R.V. Quorum sensing regulation and inhibition of exoenzyme production and biofilm formation in the food spoilage bacteria Pseudomonas psychrophila PSPF19. Food Biotechnol. 2014, 28, 293–308.
- Machado, I.; Silva, L.R.; Giaouris, E.D.; Melo, L.F.; Simões, M. Quorum sensing in food spoilage and natural-based strategies for its inhibition. Food Res. Int. 2020, 127, 108754.
- Yuan, L.; Wang, N.; Sadiq, F.A.; He, G. RNA sequencing reveals the involvement of quorum sensing in dairy spoilage caused by psychrotrophic bacteria. LWT 2020, 127, 109384.
- Li, T.; Wang, D.; Ren, L.; Mei, Y.; Ding, T.; Li, Q.; Chen, H.; Li, J. Involvement of exogenous N-acyl-homoserine lactones in spoilage potential of Pseudomonas fluorescens isolated from refrigerated turbot. Front. Microbiol. 2019, 10, 2716.
- Hassan, S.; Ahmad, T.; Bashir, M.; Kiran, G.S.; Selvin, J. Novel Perspectives on the Quorum Sensing Inhibitors (QSIs)/Quorum Quenchers (QQs) in Food Preservation and Spoilage. In Implication of Quorum Sensing and Biofilm Formation in Medicine, Agriculture and Food Industry; Bramhachari, P.V., Ed.; Springer: Singapore, 2019; pp. 269–298.
- Bai, A.J.; Vittal, R.R. Quorum sensing inhibitory and anti-biofilm activity of essential oils and their in vivo efficacy in food systems. Food Biotechnol. 2014, 28, 269–292.
- Shobharani, P.; Agrawal, R. Interception of quorum sensing signal molecule by furanone to enhance shelf life of fermented milk. Food Cont. 2010, 21, 61–69.
- Myszka, K.; Tomaś, N.; Wolko, Ł.; Szwengiel, A.; Grygier, A.; Nuc, K.; Majcher, M. In situ approaches show the limitation of the spoilage potential of Juniperus phoenicea L. essential oil against cold-tolerant Pseudomonas fluorescens KM24. Appl. Microbiol. Biotechnol. 2021, 105, 4255–4268.
- Wang, Y.; Feng, L.; Lu, H.; Zhu, J.; Kumar, V.; Liu, X. Transcriptomic analysis of the food spoilers Pseudomonas fluorescens reveals the antibiofilm of carvacrol by interference with intracellular signaling processes. Food Cont. 2021, 127, 108115.
- Wang, Y.; Wang, Y.; Chen, J.; Koseki, S.; Yang, Q.; Yu, H.; Fu, L. Screening and preservation application of quorum sensing inhibitors of Pseudomonas fluorescens and Shewanella baltica in seafood products. LWT 2021, 149, 111749.
- Gill, C.O.; Suisted, J.R. The effects of temperature and growth rate on the proportion of unsaturated fatty acids in bacterial lipids. J. Gen. Microbiol. 1978, 104, 31–36.
- Neumeyer, K.; Ross, T.; McMeekin, T.A. Development of a predictive model to describe the effects of temperature and water activity on the growth of spoilage pseudomonads. Int. J. Food Microbiol. 1997, 38, 45–54.
- Stoops, J.; Maes, P.; Claes, J.; Van Campenhout, L. Growth of Pseudomonas fluorescens in modified atmosphere packaged tofu. Lett. Appl. Microbiol. 2012, 54, 195–202.
- Nicodeme, M.; Grill, J.P.; Humbert, G.; Gaillard, J.L. Extracellular protease activity of different Pseudomonas strains: Dependence of proteolytic activity on culture conditions. J. Appl. Microbiol. 2005, 99, 641–648.
- Law, B.A.; Andrews, A.T.; Sharpe, M.E. Gelation of ultra-high-temperature-sterilized milk by proteases from a strain of Pseudomonas fluorescens isolated from raw milk. J. Dairy Res. 1977, 44, 145–148.
- De Jonghe, V.; Coorevits, A.; Van Hoorde, K.; Messens, W.; Van Landschoot, A.; De Vos, P.; Heyndrickx, M. Influence of storage conditions on the growth of Pseudomonas species in refrigerated raw milk. Appl. Environ. Microbiol. 2011, 77, 460–470.
- Stoeckel, M.; Lidolt, M.; Achberger, V.; Glück, C.; Krewinkel, M.; Stressler, T.; von Neubeck, M.; Wenning, M.; Scherer, S.; Fischer, L.; et al. Growth of Pseudomonas weihenstephanensis, Pseudomonas proteolytica and Pseudomonas sp. in raw milk: Impact of residual heat-stable enzyme activity on stability of UHT milk during shelf-life. Int. Dairy J. 2016, 59, 20–28.
- Volk, V.; Glück, C.; Leptihn, S.; Ewert, J.; Stressler, T.; Fischer, L. Two heat resistant endopeptidases from Pseudomonas species with destabilizing potential during milk storage. J. Agric. Food Chem. 2018, 67, 905–915.
- Machado, S.G.; da Silva, F.L.; Bazzolli, D.M.; Heyndrickx, M.; Costa, P.M.D.A.; Vanetti, M.C.D. Pseudomonas spp. and Serratia liquefaciens as predominant spoilers in cold raw milk. J. Food Sci. 2015, 80, M1842–M1849.
- Yuan, L.; Sadiq, F.A.; Liu, T.J.; Li, Y.; Gu, J.S.; Yang, H.Y.; He, G.Q. Spoilage potential of psychrotrophic bacteria isolated from raw milk and the thermo-stability of their enzymes. J. Zhejiang Univ. Sci. B 2018, 19, 630–642.
- Baruzzi, F.; Lagonigro, R.; Quintieri, L.; Morea, M.; Caputo, L. Occurrence of non-lactic acid bacteria populations involved in protein hydrolysis of cold-stored high moisture Mozzarella cheese. Food Microbiol. 2012, 30, 37–44.
- Scatamburlo, T.M.; Yamazi, A.K.; Cavicchioli, V.Q.; Pieri, F.A.; Nero, L.A. Spoilage potential of Pseudomonas species isolated from goat milk. J. Dairy Sci. 2015, 98, 759–764.
- Meng, L.; Liu, H.; Dong, L.; Zheng, N.; Xing, M.; Zhang, Y.; Zhao, S.; Wang, J. Identification and proteolytic activity quantification of Pseudomonas spp. isolated from different raw milks at storage temperatures. J. Dairy Sci. 2018, 101, 2897–2905.
- Ottaviani, F.; Disegna, L. Muffe e lieviti nei prodotti e negli ambienti caseari. Latte 1987, 12, 779–811.
- Quintieri, L.; Caputo, L.; De Angelis, M.; Fanelli, F. Genomic Analysis of Three Cheese-Borne Pseudomonas lactis with Biofilm and Spoilage-Associated Behavior. Microorganisms 2020, 8, 1208.
- Martin, N.H.; Murphy, S.C.; Ralyea, R.D.; Wiedmann, M.; Boor, K.J. When cheese gets the blues: Pseudomonas fluorescens as the causative agent of cheese spoilage. J. Dairy Sci. 2011, 94, 3176–3183.
- Carrascosa, C.; Millán, R.; Jaber, J.R.; Lupiola, P.; del Rosario-Quintana, C.; Mauricio, C.; Sanjuán, E. Blue pigment in fresh cheese produced by Pseudomonas fluorescens. Food Cont. 2015, 54, 95–102.
- da Silva Rodrigues, R.; Machado, S.G.; de Carvalho, A.F.; Nero, L.A. Pseudomonas sp. as the causative agent of anomalous blue discoloration in Brazilian fresh soft cheese (Minas Frescal). Int. Dairy J. 2021, 117, 105020.
- Carminati, D.; Bonvini, B.; Rossetti, L.; Zago, M.; Tidona, F.; Giraffa, G. Investigation on the presence of blue pigment-producing Pseudomonas strains along a production line of fresh mozzarella cheese. Food Cont. 2019, 100, 321–328.
- Cantoni, C.; Soncini, G.; Milesi, S.; Cocolin, L.; Iacumin, L.; Comi, G. Colorazioni anomale e rigonfiamento di formaggi fusi e mozzarelle. Ind. Aliment. 2006, 45, 276–281.
- Evanowski, R.L.; Reichler, S.J.; Kent, D.J.; Martin, N.H.; Boor, K.J.; Wiedmann, M. Pseudomonas azotoformans causes gray discoloration in HTST fluid milk. J. Dairy Sci. 2017, 100, 7906–7909.
- Kornacki, J.L.; Flowers, R.S.; Bradley, J.R.L. Microbiology of Butter. In Applied Dairy Microbiology, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001; pp. 127–150.
- Deeth, H.C.; Fitz-Gerald, C.H. Lipolytic enzymes and hydrolytic rancidity. In Advanced Dairy Chemistry Volume 2 Lipids; Springer: Boston, MA, USA, 2006; pp. 481–556.
- Samaržija, D.; Zamberlin, Š.; Pogačić, T. Psychrotrophic bacteria and their negative effects on milk and dairy products quality. Mljekarstvo: J. Dairy Prod. Proc. Imp. 2012, 62, 77–95.
- Kumar, H.; Franzetti, L.; Kaushal, A.; Kumar, D. Pseudomonas fluorescens: A potential food spoiler and challenges and advances in its detection. Ann. Microbiol. 2019, 69, 873–883.
- Corsetti, A.; Rossi, J.; Gobbetti, M. Interactions between yeasts and bacteria in the smear surface-ripened cheeses. Int. J. Food Microbiol. 2001, 69, 1–10.
- Fox, P.F.; Stepaniak, L. Isolation and some properties of extracellular heat-stable lipases from Pseudomonas fluorescens strain AFT 36. J. Dairy Res. 1983, 50, 77–89.
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology; Springer: New York, NY, USA, 2005.
- McPhee, J.D.; Griffiths, M.W. Pseudomonas spp. In Encyclopaedia of Dairy Sciences; Roginski, H., Fuquay, W.J., Fox, F.P., Eds.; Academic Press: New York, NY, USA, 2002; Volume 4, pp. 2340–2350.
- Hammad, A.M. Spoilage potential of Pseudomonas spp. isolated from domiati cheese. Assiut Vet. Med. J. 2015, 61, 18–23.
- Farkye, N.Y.; Vedamuthu, E.R. Microbiology of soft cheeses. In Dairy Microbiology Handbook. The Microbiology of Milk and Milk Products; Robinson, R.K., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2002; pp. 479–513.
- Paludetti, L.F.; Kelly, A.L.; Gleeson, D. Effect of thermoresistant protease of Pseudomonas fluorescens on rennet coagulation properties and proteolysis of milk. J. Dairy Sci. 2020, 103, 4043–4055.
- Stuknytė, M.; Decimo, M.; Colzani, M.; Silvetti, T.; Brasca, M.; Cattaneo, S.; Aldini, G.; De Noni, I. Extracellular thermostable proteolytic activity of the milk spoilage bacterium Pseudomonas fluorescens PS19 on bovine caseins. J. Dairy Sci. 2016, 99, 4188–4195.
- D’Incecco, P.; Brasca, M.; Rosi, V.; Morandi, S.; Ferranti, P.; Picariello, G.; Pellegrino, L. Bacterial proteolysis of casein leading to UHT milk gelation: An applicative study. Food Chem. 2019, 292, 217–226.
- Crudden, A.; Fox, P.F.; Kelly, A.L. Factors affecting the hydrolytic action of plasmin in milk. Int. Dairy J. 2005, 15, 305–313.
- Mara, O.; Roupie, C.; Duffy, Y.A.; Kelly, A.L. The Curd-forming Properties of Milk as affected by the action of plasmin. Int. Dairy J. 1998, 8, 807–812.
- Liu, M.; Wang, H.; Griffiths, M.W. Regulation of alkaline metalloprotease promoter by N-acyl homoserine lactone quorum sensing in Pseudomonas fluorescens. J. Appl. Microbiol. 2007, 103, 2174–2184.
- Liao, C.H.; McCallus, D.E. Biochemical and genetic characterization of an extracellular protease from Pseudomonas fluorescens CY091. Appl. Environ. Microbiol. 1998, 64, 914–921.
- Quintieri, L.; Pistillo, B.R.; Caputo, L.; Favia, P.; Baruzzi, F. Bovine lactoferrin and lactoferricin on plasma-deposited coating against spoilage Pseudomonas spp. Inn. Food Sci. Emerg. Technol. 2013, 20, 215–222.
- Caputo, L.; Quintieri, L.; Bianchi, D.M.; Decastelli, L.; Monaci, L.; Visconti, A.; Baruzzi, F. Pepsin-digested bovine lactoferrin prevents Mozzarella cheese blue discoloration caused by Pseudomonas fluorescens. Food Microbiol. 2015, 46, 15–24.
- Ledenbach, L.H.; Marshall, R.T. Microbiological spoilage of dairy products. In Compendium of the Microbiological Spoilage of Food and Beverages Industries; Doyle, M.P., Sperber, W.H., Eds.; Springer: New York, NY, USA, 2009; pp. 41–68.
- Morales, P.; Fernandez-Garcia, E.; Nunez, M. Production of volatile compounds in cheese by Pseudomonas fragi strains of dairy origin. J. Food Prot. 2005, 68, 1399–1407.
- Deeth, H.C. Lipases from Milk and Other Sources. In Agents of Change. Food Engineering Series; Kelly, A.L., Larsen, L.B., Eds.; Springer: Cham, Switzerland, 2021.
- Decimo, M.; Brasca, M.; Ordóñez, J.A.; Cabeza, M.C. Fatty acids released from cream by psychrotrophs isolated from bovine raw milk. Int. J. Dairy Technol. 2017, 70, 339–344.
- Deeth, H.C.; Fitzgerald, C.H. Lipolytic enzymes and hydrolytic rancidity in milk and milk products. In Developments in Dairy Chemistry; Fox, P.F., Ed.; Elsevier: Amsterdam, The Netherlands; Applied Science Publishers: London, UK, 1983; Volume 2, pp. 195–239.
- Ghoddusi, H.; Özer, B. Microbiology of cream, butter, ice cream and related products. In Dairy Microbiology and Biochemistry–Recent Developments; Ozer, B., Akdemir-Evrendilek, G., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 245–270.
- Soncini, G.; Marchisio, E.; Cantoni, C. Causes of chromatic alterations in Mozzarella cheese. Ind. Alim. 1998, 37, 850–855.
- Franzetti, L.; Scarpellini, M. Characterisation of Pseudomonas spp. isolated from foods. Annals Microbiol. 2007, 57, 39–47.
- Palleroni, N.J.; Genus, I. Pseudomonas Migula 1894. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Eds.; The Proteobacteria, Part B, The Gammaproteobacteria; Springer: New York, NY, USA, 2005; Volume 2, pp. 323–379.
- RASFF. Rapid Alert System for Food and Feed. Annual Report. 2010. Available online: https://op.europa.eu/en/publication-detail/-/publication/7de58882-f5c5-4e28-b8b5-0ebf9836dbdf/language-en/format-PDF/source-174744260 (accessed on 12 December 2020).
- Nogarol, C.; Acutis, P.L.; Bianchi, D.M.; Maurella, C.; Peletto, S.; Gallina, S.; Adriano, D.; Zuccon, F.; Borrello, S.; Caramelli, M.; et al. Molecular characterization of Pseudomonas fluorescens isolates involved in the Italian “blue mozzarella” event. J. Food Prot. 2013, 76, 500–504.
- Andreani, N.A.; Martino, M.E.; Fasolato, L.; Carraro, L.; Montemurro, F.; Mioni, R.; Bordin, P.; Cardazzo, B. Tracking the blue: A MLST approach to characterise the Pseudomonas fluorescens group. Food Microbiol. 2014, 39, 116–126.
- Quintieri, L.; Fanelli, F.; Zühlke, D.; Caputo, L.; Logrieco, A.F.; Albrecht, D.; Riedel, K. Biofilm and pathogenesis-related proteins in the foodborne P. fluorescens ITEM 17298 with distinctive phenotypes during cold storage. Front. Microbiol. 2020, 11, 991.
- Fanelli, F.; Liuzzi, V.C.; Quintieri, L.; Mulè, G.; Baruzzi, F.; Logrieco, A.F.; Caputo, L. Draft genome sequence of Pseudomonas fluorescens strain ITEM 17298, associated with cheese spoilage. Genome Announc. 2017, 5, e01141–e01217.
- European Commission. Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the transparency and sustainability of the EU risk assessment in the food chain. Off. J. Eur. Comm. 2019, L231, 1–28.
- Teh, K.H.; Flint, S.; Palmer, J.; Andrewes, P.; Bremer, P.; Lindsay, D. Biofilm—An unrecognised source of spoilage enzymes in dairy products? Int. Dairy J. 2014, 34, 32–40.
- Wettstadt, S.; Llamas, M.A. Role of regulated proteolysis in the communication of bacteria with the environment. Front. Mol. Biosci. 2020, 7, 586497.
- Bai, A.J.; Vittal, R.R. Bacterial quorum sensing and food industry. Compr. Rev. Food Sci. Food Saf. 2011, 10, 183–193.
- McCarthy, C.N.; Woods, R.G.; Beacham, I.R. Regulation of the aprX-lipA operon of Pseudomonas fluorescens B52: Differential regulation of the proximal and distal genes, encoding protease and lipase, by ompR-envZ. FEMS Microbiol. Lett. 2004, 241, 243–248.
- Machado, S.G.; Baglinière, F.; Marchand, S.; Van Coillie, E.; Vanetti, M.C.; De Block, J.; Heyndrickx, M. The biodiversity of the microbiota producing heat-resistant enzymes responsible for spoilage in processed bovine milk and dairy products. Front. Microbiol. 2017, 8, 302.
- Liu, X.; Ji, L.; Wang, X.; Li, J.; Zhu, J.; Sun, A. Role of RpoS in stress resistance, quorum sensing and spoilage potential of Pseudomonas fluorescens. Int. J. Food Microbiol. 2018, 270, 31–38.
- Ammor, M.S.; Flórez, A.B.; van Hoek, A.H.; de Los Reyes-Gavilán, C.G.; Aarts, H.J.; Margolles, A.; Mayo, B. Molecular characterization of intrinsic and acquired antibiotic resistance in lactic acid bacteria and bifidobacteria. J. Mol. Microbiol. Biotechnol. 2008, 14, 6–15.
- Rosenau, F.; Jaeger, K.E. Bacterial lipases from Pseudomonas: Regulation of gene expression and mechanisms of secretion. Biochimie 2000, 82, 1023–1032.
- Prigent-Combaret, C.; Brombacher, E.; Vidal, O.; Ambert, A.; Lejeune, P.; Landini, P.; Dorel, C. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 2001, 183, 7213–7223.
- Sacherer, P.; Défago, G.; Haas, D. Extracellular protease and phospholipase C are controlled by the global regulatory gene gacA in the biocontrol strain Pseudomonas fluorescens CHA0. FEMS Microbiol. Lett. 1994, 116, 155–160.
- Quintieri, L.; Fanelli, F.; Caputo, L. Antibiotic resistant Pseudomonas spp. spoilers in fresh dairy products: An underestimated risk and the control strategies. Foods 2019, 8, 372.
- Armes, A.C.; Buchan, A. Cyclic di-GMP is integrated into a hierarchical quorum sensing network regulating antimicrobial production and biofilm formation in Roseobacter clade member Rhodobacterales Strain Y4I. Front. Mar. Sci. 2021, 8, 681551.
- Cude, W.N.; Buchan, A. Acyl-homoserine lactone-based quorum sensing in the Roseobacter clade: Complex cell-to-cell communication controls multiple physiologies. Front. Microbiol. 2013, 4, 336.
- Lan, L.; Murray, T.S.; Kazmierczak, B.I.; He, C. Pseudomonas aeruginosa OspR is an oxidative stress sensing regulator that affects pigment production, antibiotic resistance and dissemination during infection. Mol. Microbiol. 2010, 75, 76–91.
- Andreani, N.A.; Carraro, L.; Zhang, L.; Vos, M.; Cardazzo, B. Transposon mutagenesis in Pseudomonas fluorescens reveals genes involved in blue pigment production and antioxidant protection. Food Microbiol. 2019, 82, 497–503.
- Reichler, S.J.; Martin, N.H.; Evanowski, R.L.; Kovac, J.; Wiedmann, M.; Orsi, R.H. A century of gray: A genomic locus found in 2 distinct Pseudomonas spp. is associated with historical and contemporary color defects in dairy products worldwide. J. Dairy Sci. 2019, 102, 5979–6000.
- Bishop, T.F.; Martin, L.W.; Lamont, I.L. Activation of a cell surface signaling pathway in Pseudomonas aeruginosa requires ClpP protease and new sigma factor synthesis. Front. Microbiol. 2017, 8, 2442.
- Wilderman, P.J.; Vasil, A.I.; Johnson, Z.; Wilson, M.J.; Cunliffe, H.E.; Lamont, I.L.; Vasil, M.L. Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect. Immun. 2001, 69, 5385–5394.
- Azzara, C.D.; Campbell, L.B. Off-flavors of dairy products. In Developments in Food Science; Off-Flavors in Foods and Beverages; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 329–374.
- Andreani, N.A.; Carraro, L.; Fasolato, L.; Balzan, S.; Lucchini, R.; Novelli, E.; Cardazzo, B. Characterisation of the thermostable protease AprX in strains of Pseudomonas fluorescens and impact on the shelf-life of dairy products: Preliminary results. Ital. J. Food Saf. 2016, 5, 6175.
- Gloria, M.B.A.; Saraiva, P.R.; Rigueira, J.C.; Brandao, S.C. Bioactive amines changes in raw and sterilised milk inoculated with Pseudomonas fluorescens stored at different temperatures. Int. J. Dairy Technol. 2011, 64, 45–51.
- Luengo, J.M.; Olivera, E.R. Catabolism of biogenic amines in Pseudomonas species. Environ. Microbiol. 2020, 22, 1174–1192.
