Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
|
Engineering, Electrical & Electronic
Silicon is the second most earth-abundant element in the earth’s crust, and the most abundant element used in modern-day technologies. Its popularity partially stems from the nontoxic and relatively unreactive nature of derivatives, such as silica and silicates, at room temperature. Consequently, the sheer volume of silicon being used has accelerated the rate of production of waste materials.
- silicon
- waste silicon
- semiconductor
- energy harvesting
- electronic waste
- thermoelectrics
- solar cell waste
1. Introduction
The increasing trend in energy generation worldwide is accelerating with a high of 162,194 TWh recorded in 2019, 21% more in comparison with a decade ago [1]. With this trend, it is imperative to explore various sources of energy, extending our options from finite natural resources and current methods of sustainable energy generation. Despite the development and advances in renewable energy sources, energy conversion is never completely efficient.
Energy generation, whether by fuel combustion or by conversion from other energy forms, usually involves a certain degree of heat loss to the environment. This heat loss becomes more pronounced at higher operating temperatures due to the large thermal difference between the source, the mechanical components, and the surroundings, promoting energy dissipation by radiation. Additionally, the waste heat produced, if not sufficiently dissipated, can be counterproductive to energy generation. For example, in a study conducted by Benghanem et al., it was shown that the efficiency of photovoltaic cells past the optimal operating temperature decreases by 0.5% with every °C increase [2]. It may then be expedient to focus on improving the energy conversion efficiencies in high-temperature applications whereby the residual heat prevents optimal performance to a larger extent.
In addition, attention should be directed to applications that not only incur the most heat loss but also utilize a large amount of fuel to further amplify economic benefits from a more efficient energy conversion. One example would be commercial aircraft, which are consuming up to 95 billion gallons of diesel in 2019 (before the coronavirus pandemic) due to the booming aviation industry, [3,4,5,6,7,8,9,10]. Furthermore, extra consideration should be taken for applications that utilize scarce material as energy sources, such as radioisotope thermoelectric generators (RTGs) powered by naturally occurring but limited radioactive oxides. To fully maximize the energy conversion processes in these applications, waste heat can be harvested and converted into electricity, which can be achieved through the use of thermoelectric generators (TEGs).
2. Materials Selection
2.1. Silicon
Silicon is the second most earth-abundant element in the earth’s crust, and the most abundant element used in modern-day technologies [116]. Its popularity partially stems from the nontoxic and relatively unreactive nature of derivatives, such as silica and silicates, at room temperature [117]. Consequently, the sheer volume of silicon being used has accelerated the rate of production of waste materials. Mainstream applications, such as electronics and semiconductors, typically require ultrapure silicon, which undergo a tedious and expensive purification process. Thermoelectric applications are also affected by defects, albeit less sensitively compared with other applications, which suggests that the cost of fabrication can be much cheaper. Intrinsically, bulk silicon has a high thermal conductivity of ~150 Wm−1 K−1 at room temperature. Fortunately, such parameters can be optimized to give a decent zT value. Several works in the recent two decades have studied the potential of elemental silicon as a thermoelectric material in various forms [118,119,120,121,122,123]. For instance, a work conducted by Bux et al. compared the performances of synthesized nanostructured bulk Si and Si0.8Ge0.2 and found that the thermal conductivity of nanostructured bulk Si could be significantly reduced and ultimately produce a zT of 0.7 at 1200 K. While in the initial days, extensive silicon purification did not pose much of a problem, the waste from the first-generation electronics boom in recent years has caused it to become a major issue. This problem is exacerbated by the exponential increase in solar cell waste [124]. For instance, it is often economically and technologically not feasible to recycle used silicon (often alloyed or doped with other elements) for delicate use, such as in the high-performance electronics industry. In addition, the process of cutting Si ingots with wire for solar cell fabrication leads to a material loss of 40% in the form of kerf [125]. Fortunately, this is where thermoelectrics can play a vital role. As a majority carrier device (as opposed to a minority carrier device, such as photovoltaics), thermoelectrics are generally less defect sensitive, and existing impurities may even be beneficial to their performance instead. Additionally, since silicon is safe to handle in comparison with other compounds composed of toxic or harmful elements, such as Pb or Te, the mass production of silicon-based thermoelectrics would not pose any concerns to human or environmental health.
2.2. Recycled Silicon
High-performance thermoelectrics can often be achieved via defect engineering, where lattice defects or impurities are deliberately introduced into the materials to improve their electrical or thermal properties. This makes thermoelectrics a perfect target for recycling (or upcycling) silicon-based electronics waste (e-waste) and other technological silicon waste. Figure 4 shows a schematic of how thermoelectrics can benefit from the existing defects of the waste material.
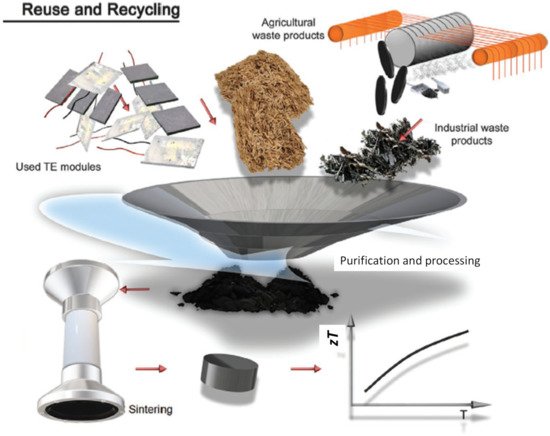
Figure 4. Schematic showing waste upcycling of industrial and agricultural waste into thermoelectrics. Figure from Bahrami et al. [125]. Copyright 2020, Wiley (Weinheim, Germany).
Recycling waste material into thermoelectrics is an area that has been explored for other elements, such as bismuth [126,127]. Cai et al. recently explored the recyclability of bismuth antimony telluride thermoelectrics and discovered that the combination of reprocessed (Bi,Sb)2Te3 scraps and nano-SiC showcased a better zT of 1.07 at 325 K even in comparison with the commercial alloy, which was 0.95. Further work conducted on the specifics of the nanocomposition yielded an improved zT of 1.33.
Similarly, the merits of upcycled silicon-based thermoelectrics has been recognized but not yet popularized, which undermines its potential in championing sustainable energy harvesting and cooling technologies. For instance, the pioneering encounter with recycled silicon thermoelectric stemmed from rice husks. Si-based compounds obtained from the valorization of rice husks were used by Bose et al. to synthesize Mg2Si [128]. However, the results then were not particularly promising with a zT of 1 × 10−4 due to the impurities that were retained in the synthesis of the compound [129]. More recently, Ran et al. discovered that upcycled silicon from sawing waste, doped with phosphorous, achieved a power factor of 32 µW cm−1 K−2 at 1273 K and, subsequently, a peak zT of 0.33 [130]. Comparatively, SiGe alloys (to be discussed later) performed with peak zT values reaching ~0.65 and ~1 in p- and n-type Si80Ge20, respectively, were consistently reported in multiple studies for temperatures of 900–950 °C.
Additionally, there is an abundance of silicon-based waste products as a result of the escalating rate of production of PV modules. Fabrication of such electronics produces by-products, such as SiC, diamond particles, metals, and oxidized silicon. While it may be intuitive to prioritize recycling precious and expensive materials, such as silver and gold, thankfully, in recent years, there has been more effort to recycle Si particles [131]. Aside from utilizing Si kerf for TE synthesis, other Si-based by-products can be purified and utilized for the synthesis of compounds, such as Mg2Si. Isoda et al. [132] and Mesaritis et al. [133] successfully utilized Si kerf as a precursor to Mg2(Si,Ge,Sn) alloys, which perform competitively for TE made from commercial silicon.
It is worth noting that although recycled silicon does not have very high average figure of merit, the wide temperature gradient made available by the high operating temperature allows for a better Carnot efficiency, resulting in decent power conversion efficiencies. Figure 5a,b shows the power factor and figure of merit of silicon thermoelectrics processed from sawing waste from recent works. Although such number is nowhere near the recorded zT of thermoelectrics, it can still provide 5–7% power conversion efficiency based on Equation (2). Further optimization of the doping for recycled silicon can present opportunities for thermoelectrics with performances competitive with popular SiGe.
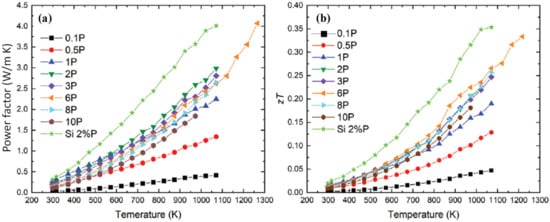
Figure 5. (a) Power factor and (b) figure of merit zT for silicon from sawing waste, with different levels of P doping. Figure from Ran et al. [130].
2.3. Silicon Germanium
Silicon germanium is a semiconducting alloy with a diamond crystal structure popularly used for high-temperature thermoelectric applications. The alloy is miscible at all ratios of SiGe but presents a challenge in consistent synthesis due to its complex phase diagram. Prior to the development of SiGe as a thermoelectric, silicon was the staple for high-temperature applications due to its abundance and nontoxicity. However, it is disadvantaged as a thermoelectric material due to its low electrical conductivity and high thermal conductivity. The conductivity of silicon can be improved through aliovalent doping of boron and phosphorus to create p-type and n-type semiconductors, respectively. However, this is insufficient to boost its performance as a thermoelectric without improvements in its thermal conductivity.
Conversely, germanium is also a semiconducting material with a small band gap of 0.66 eV. It exhibits good electrical conductivity and low thermal conductivity, making it a promising thermoelectric option for low-temperature applications. However, germanium is much less abundant than silicon at 1.5 parts per million and is also rather costly [134]. Hence, it is more frequently used as an alloying component to other thermoelectric materials, such as SiGe and GeTe.
The addition of Ge to a Si matrix introduces mass fluctuations, varying the lattice parameters as well. Dismukes modelled the relation between the lattice constant and the percentage Ge in 1964, slightly modifying Vegard’s law, as shown in Equation (6) [135,136].
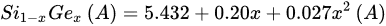
The mass fluctuations created due to the difference in atomic size of silicon and germanium scatters short-wave acoustic phonons, which contribute largely to the material’s thermal conductivity. A paper by Virginia Semiconductor compares the electronic characteristics of Si, Ge, and some compositions of SiGe [137]. The electrical properties of the Si1−xGex alloys vary proportionately to the amount of germanium added to the silicon matrix, reducing the resistivity and thermal conductivity while increasing the drift mobility of charge carriers.
The ratio of Si to Ge was studied for optimal composition to maximize the scattering of acoustic phonons, and it was found that a 20% substitution of Ge exhibited a significant decrease in thermal conductivity to ~9 W cm−1 K, and a further addition of Ge contributed insignificantly [138]. Additionally, Ge is about 100 times more costly as compared with Si [139]. Therefore, minimizing the required amount for substitution would be financially beneficial. The alloying of silicon and germanium in different ratios allows for the fabrication of thermoelectric materials with various operating temperatures due to the changes in the band gap. This offers a solution to silicon’s shortcoming as a thermoelectric, presenting us with SiGe as an option for thermoelectric materials at high-temperature applications.
Adding on the promise of intrinsic SiGe, multiple modifications have been studied to further enhance the performance of this alloy, including nanostructuring, modulation doping, and various methods of synthesis. SiGe exhibits a complex phase diagram, as illustrated in Figure 6. There is a large separation between the solidus and liquidus lines, suggesting that the composition of the SiGe alloy is sensitive to temperature and the amount of Ge added.
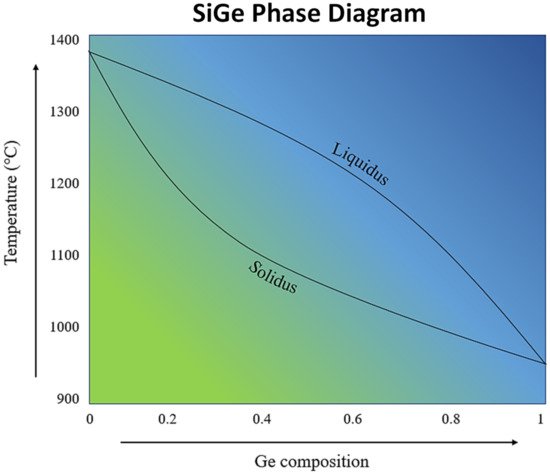
Figure 6. Phase diagram of SiGe. Figure reproduced with permission from “Towards a Fully Functional Integrated Photonic–Electronic Platform via a Single SiGe Growth Step” from Littlejohns et al., 2016 [140].
According to a recent review by Basu et al., the solid-state synthesis of SiGe through mechanical alloying, followed by densification by hot pressing or spark plasma sintering, is the key to consistent replication of desired stoichiometry. Mechanical alloying is also a simple and economical method to produce homogeneous alloys with consistent grain sizes. Hot isostatic pressing utilizes a combination of temperature and pressure to densify the ball-milled powder. The alternative to hot pressing is spark plasma sintering (SPS), whereby a high current is applied through the sample, heating the sample while under compression. In comparison with SPS, hot pressing is less favored as it encourages grain growth. Similar to silicon, p-type SiGe is most commonly synthesized by doping with boron and n-type with phosphorus.
A popular strategy for enhancing the thermoelectric performance is modulation doping. Compared with uniform doping, modulation doping is the selective addition of dopants in spatially segregated areas, as shown in Figure 7. The clustered-doping method promotes higher charge mobility as the ionized dopants will be segmented into the designated areas and limit the electrostatic scattering of charges [141]. Hence, it can be expected that modulation doping will improve the power factor. However, it is also important to consider that the dopants have their own thermal conductivity. The higher thermal conductivity of the introduced precipitates limits the improvement of zT by the increase in power factor.
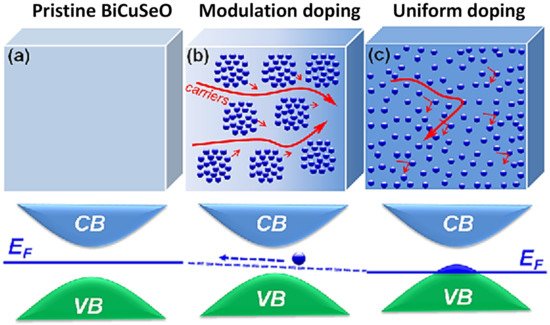
Figure 7. Schematic showing a carrier transport in (a) pristine BiCuSeO, (b) modulation-doped and (c) uniformly doped samples. Figure reproduced with permission from “High Thermoelectric Performance Realized in a BiCuSeO System by Improving Carrier Mobility through 3D Modulation Doping” from Pei et al., 2014 [142].
For example, a previous work conducted by Yu et al. demonstrated their three-dimensional modulation doping approach using a two-phase nanocomposite of different types of nanograins. In their study, an improvement of 40% was realized in the power factor of Si80Ge20 doped to 30% with Si100B, forming the composite (Si80Ge20)0.7(Si100B5)0.3 in comparison with Si86Ge14B1.5. Conversely, for the n-type Si84Ge16P0.6, the modulation-doped composite (Si80Ge20)0.8(Si100P3)0.2 exhibited an improvement in power factor by 20%. Despite the increase in power factor for both samples, the zTs did not improve due to the high thermal conductivity of the Si nanoparticles. Yu elaborated that the use of modulation doping strategies will inevitably bring about an increase in the electronic component as charge carriers are thermal carriers and also an increase in the lattice component when the nanoparticles have a higher thermal conductivity [143]. In turn, more attention should be directed to the reduction of the lattice component. This further modification was also discussed in his work.
2.4. Other Silicon-Based Compounds
Aside from SiGe, many other Si-based compounds have been of interest for thermoelectric use due to silicon’s crust abundance and nontoxicity. Each of these has its own merits and shortcomings, which will be briefly discussed in this section. To date, the compounds that have gained traction in the thermoelectric field are metal silicides, more specifically for alkali, alkaline, and transition metals. However, the optimal operating temperatures for each of them vary according to the alloying composition.
Ru2Si3 was once considered a potentially competitive high-temperature thermoelectric material, alongside SiGe alloys. Its interesting band structure suggested an initial extrapolated zT 50–300% higher than that of SiGe, assuming that the carrier mobility of Ru2Si3 trends similarly to that of silicon [144,145]. Sawade et al. synthesized undoped Ru2Si3, which boasted a zT of 0.32 at 900 K [146]. Despite further work on the compound, such as Mn doping, breakthroughs since then have been limited due to a lack of suitable dopants and scarcity concerns of elemental Ru [147].
Most other metal silicides are observed to be suitable for medium temperature applications. There is a wide array of transition metal silicides that have been developed for thermoelectric use, such as MnSix, CrSi2, WSi2, and MoSi2. Within this category, good thermoelectric performance was exhibited by the higher manganese silicide (HMS) compounds, which exhibited a zT of 0.5–0.7 at 500 °C [148].
The superior performance of HMS partly stems from MnSi, which introduces energy barriers and improves carrier charge transport. In addition, HMS alloys are mechanically robust and highly resistant to oxidation, making their fabrication into module easier. Many studies have been conducted to further improve the performance of HMS, such as nanostructuring, Ge substitution, Al substitution, and nanoinclusions, with some degree of success. However, more studies could be conducted in improving the power factor of HMS to further boost zT.
Likewise, recycled silicon has been studied for the synthesis of Mg2Si. Akasaka et al. and Honda et al. achieved a zT of 0.5–0.6 at 800–860 K, and Isoda et al. and Mesaritis et al. synthesized Mg2(Si,Ge,Sn) thermoelectrics with purified Si kerf and reported a decent zT. The values achieved are competitive with pristinely synthesized thermoelectrics, and this opens up an opportunity for cheaper and more sustainable materials.
Si-based materials have exhibited great potential in waste heat harvesting at medium to high temperatures. The choice of material for TE module fabrication and subsequently deployment in commercial aviation or aerospace application warrants additional consideration of other aspects of the material to ensure that the device performs with optimal power efficiency for a reasonable lifetime.
This entry is adapted from the peer-reviewed paper 10.3390/cryst12030307
This entry is offline, you can click here to edit this entry!
