Fusarium wilt disease is leading threat to watermelon yield and quality. Different cultivation cropping systems have been reported as safe and efficient methods to control watermelon Fusarium wilt. However, the role of salicylic acid (SA) in watermelon resistance to Fusarium wilt in these different cultivation systems remains unknown. RNA-seq and qRT-PCR are used to study the effect of SA biosynthesis on improving watermelon health, demonstrating how it may be responsible for Fusarium wilt resistance under continuous monocropping and oilseed rape rotation systems. Results indicated that the expression of the CIPALs genes was key to SA accumulation in watermelon plants. The NPR family genes may have played different roles in responding to the SA signal.
- salicylic acid
- watermelon
- Fusarium wilt
- resistance
1. Introduction
2. Comparison of Disease Incidence, Watermelon Root Morphological Phenotype, and Physiological Indexes
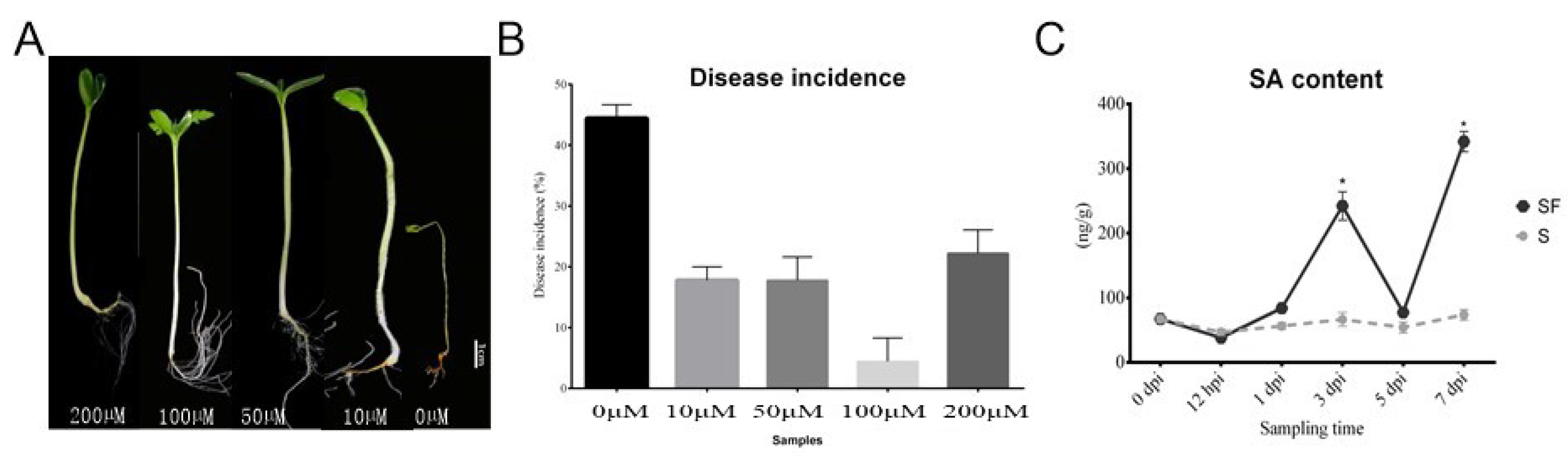
Figure 1. The effect of salicylic acid on watermelon resistance to Fusarium wilt in the pot experiment: (A) The phenotypes of watermelon seedling after SA application at different concentrations; (B) comparison of disease incidence after different concentrations of exogenous SA were applied; (C) comparison of SA content in watermelon treated with FON. S: control (Zaojia 8424, sensitive cultivar); SF: Zaojia 8424, sensitive cultivar + FON. Note: 0 dpi (before treatment); 12 hpi (12 h postinoculation); 1 dpi (1 day postinoculation); 3 dpi (3 days postinoculation); 5 dpi (5 days postinoculation); 7 dpi (7 days postinoculation). Data are expressed as mean ± SE (n = 3). Multiple t tests of two-way ANOVA (* p ≤ 0.0001).
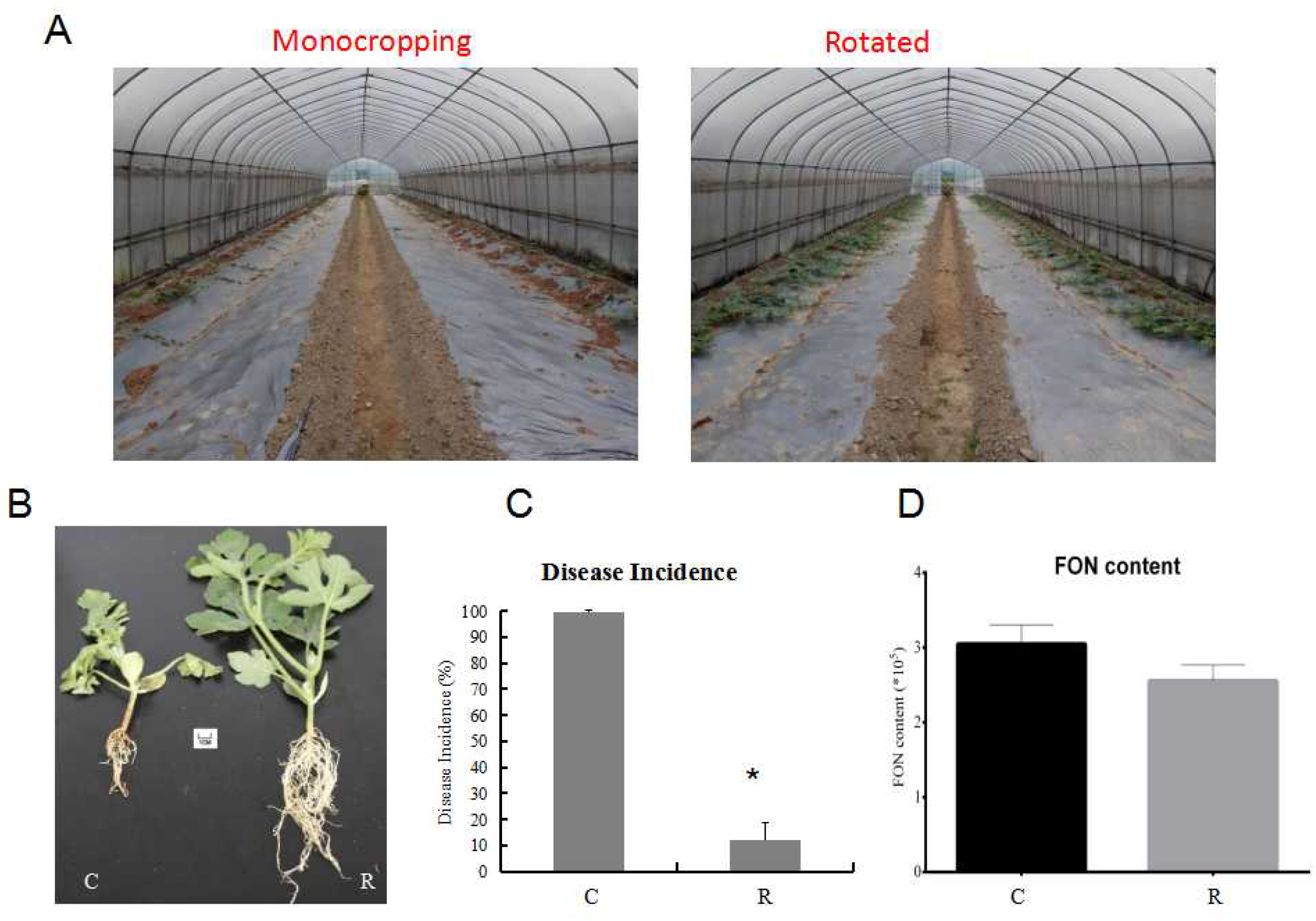
Figure 2. Comparison of watermelon growth and disease incidence under different growth conditions in the field experiment. (A) Comparison of watermelon plants’ morphology under monocropping and rotated cropping systems; (B) the phenotypes of watermelon seedlings under different systems; (C) the disease incidence under different growth systems; (D) FON biomass in two cropping systems. C: continuous watermelon monocropping; R: oilseed rape rotation cropping. Data are expressed as mean ± SE (n = 3). Student’s T-test (* p ≤ 0.05).
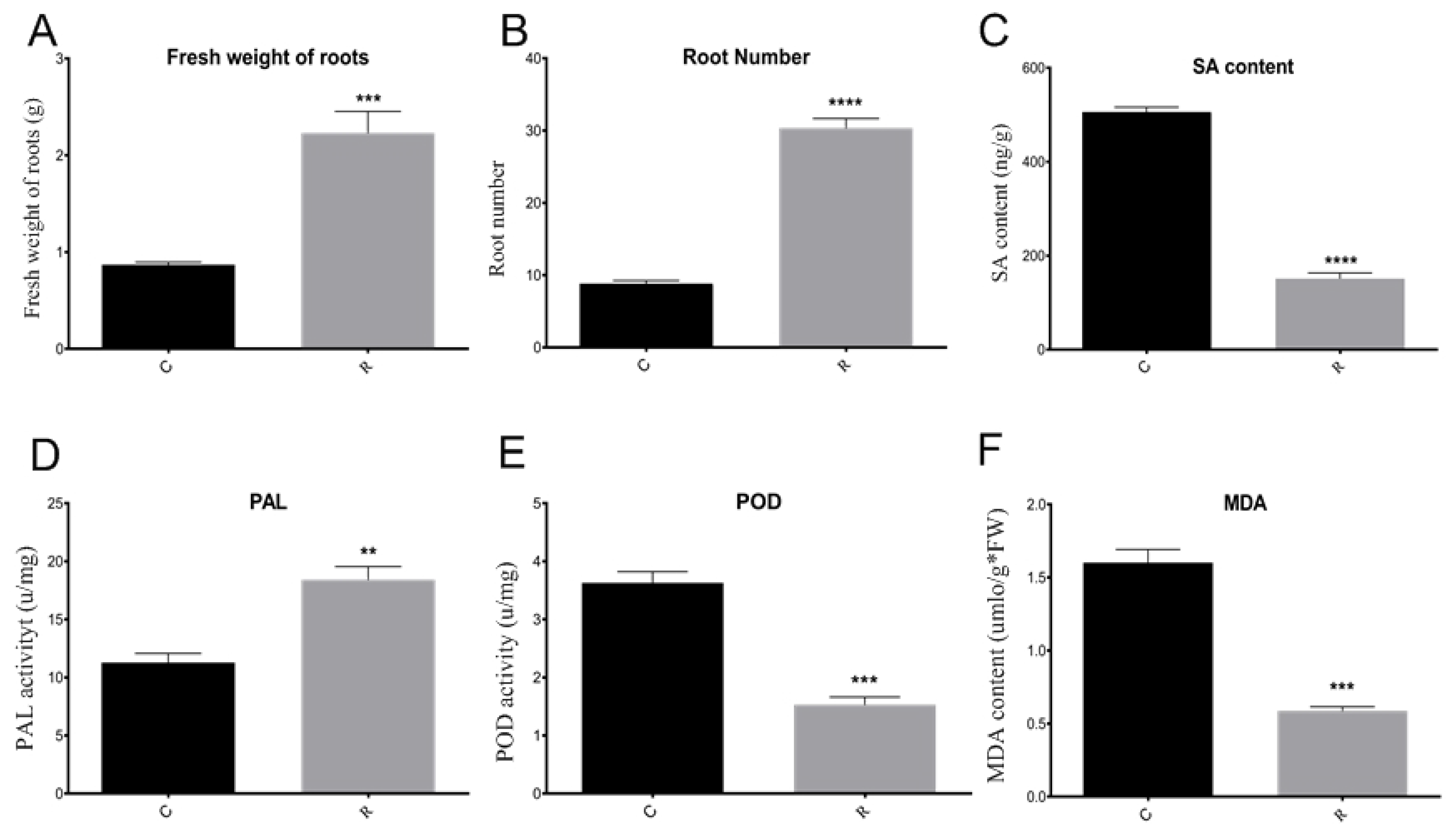
Figure 3. Comparison of watermelon physiological and biochemical indexes. (A) Comparison of fresh root weights in different samples; (B) comparison of root number in different samples; (C) comparison of SA contents in different samples; (D) comparison of PAL enzyme activity in different samples; (E) comparison of POD enzyme activity in different samples; (F) comparison of MDA content in different samples. C: continuous watermelon monocropping; R: rotated with oilseed rape cropping; POD: peroxidase; PAL: phenylalanine ammonia-lyase; MDA: malondialdehyde. Three biological replicates per samples were analyzed. Data are expressed as mean ± SE (n = 3). Student’s t-test (** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001).
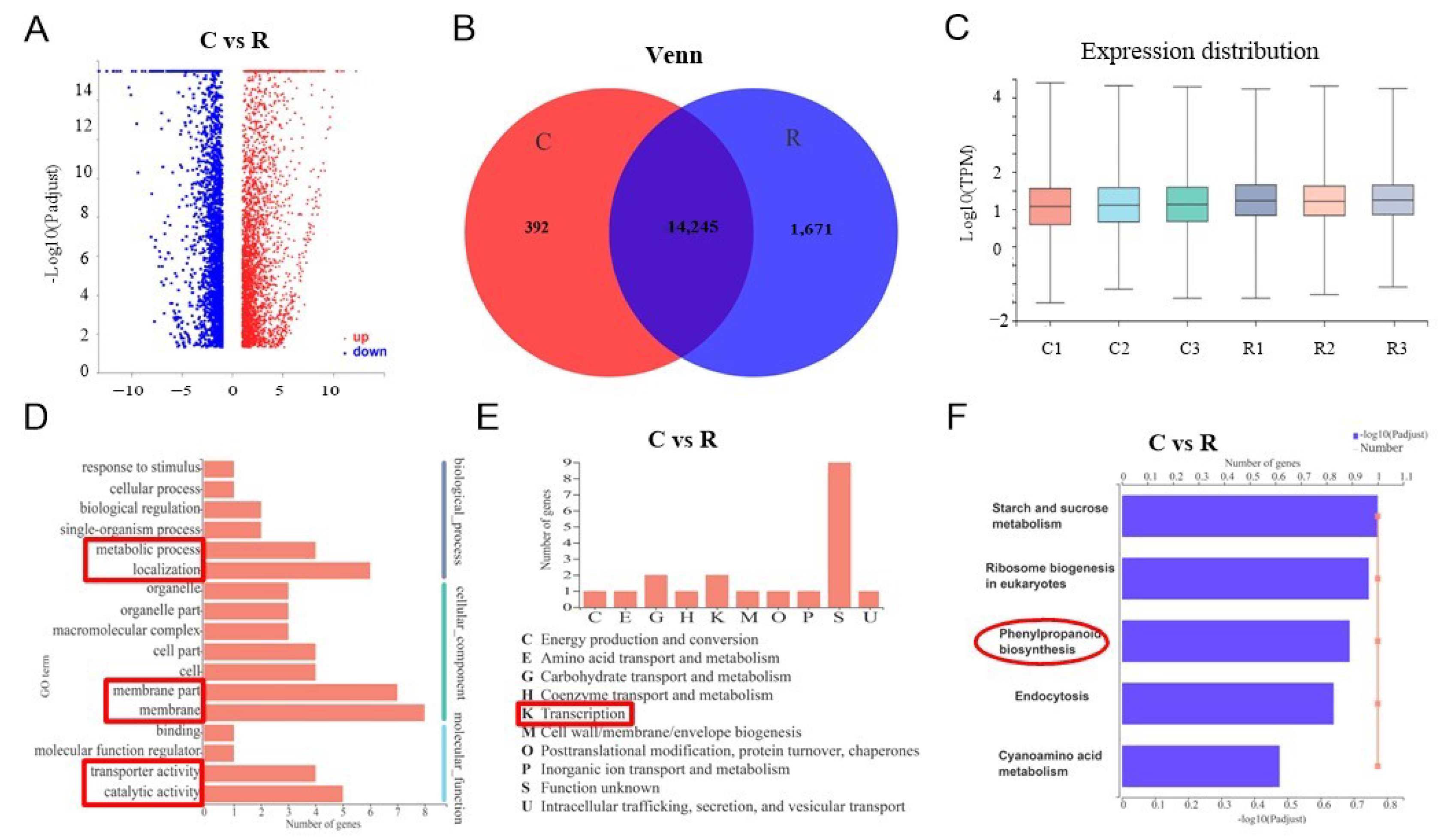
3. Transcriptome Profiling of Watermelon Roots under Different Growth Conditions

4. Expression Verification of SA Biosynthesis Genes
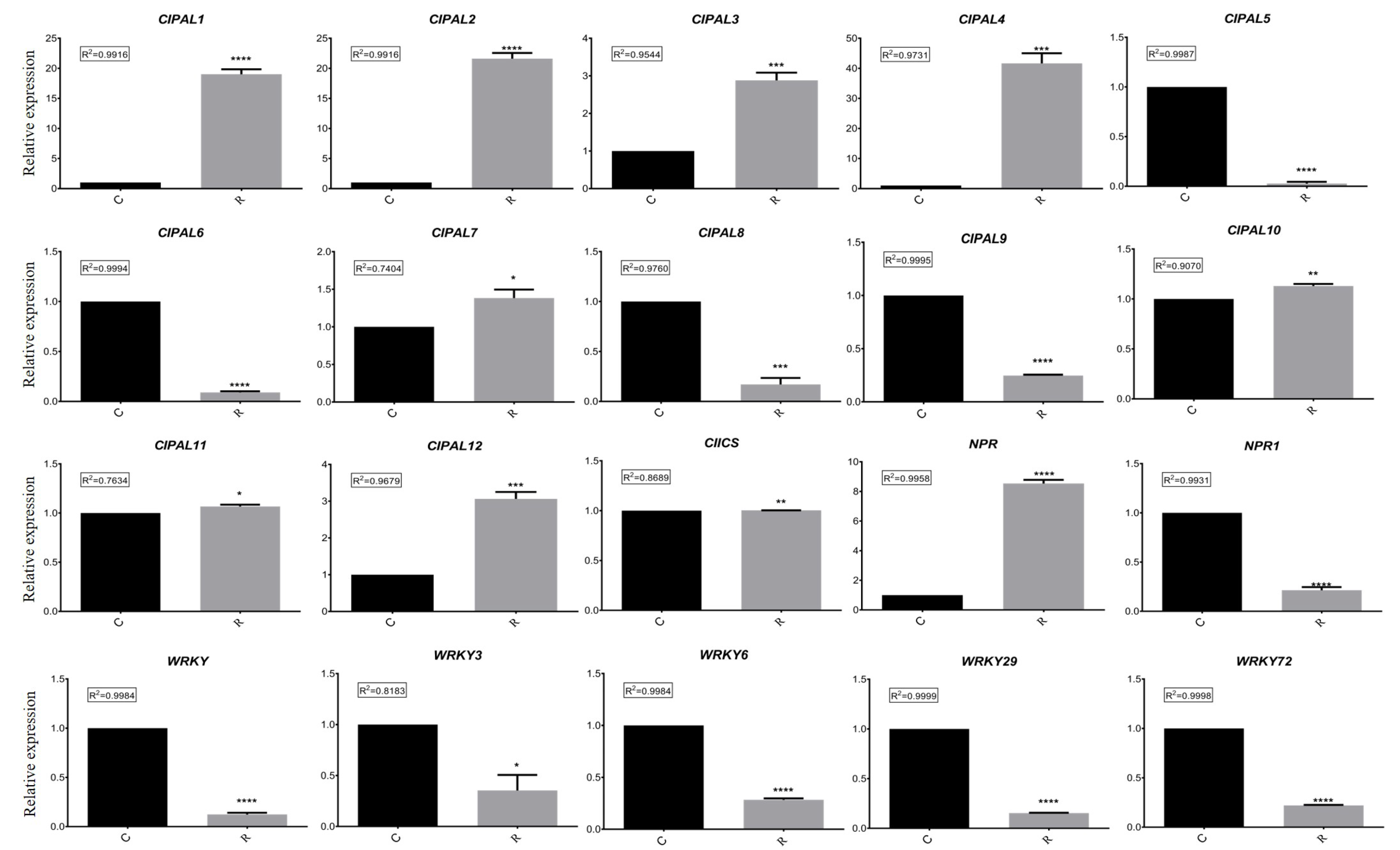
Figure 6. Comparison analysis of relative expressions of 20 candidate genes in different samples using RT-qPCR. C: continuous watermelon monocropping; R: oilseed rape rotation cropping. Three biological replicates per samples were analyzed. Data are expressed as mean ± SE (n = 3). Student’s t-test (** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001).
5. Bioinformatics Analysis of Candidate Genes
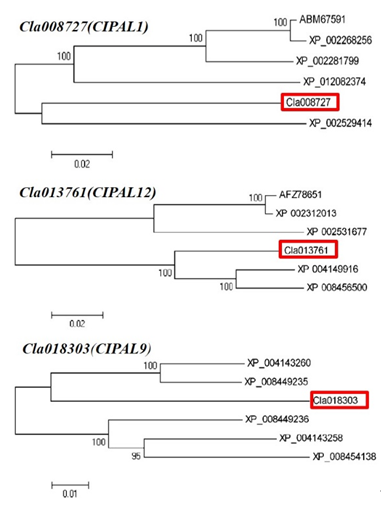
Three candidate SA synthesis genes were selected for protein homology analysis using NCBI BLAST. The results showed that the Cla008727 protein exhibited a high level of similarity with ABM 67591.1 | phenylalanine ammonia-lyase (Vitis vinifera L.) and XP_ 012082374.1 | phenylalanine ammonia-lyase (Jatropha curcas L.). The Cla013761 protein demonstrated a high level of similarity with Cucumis sativus L., Ricinus communis L., Populus trichocarpa (Torr. & Gray), Populus tomentosa Carrière, and Cucumis melo L. phenylalanine ammonia-lyase. In addition, the Cla 018303 protein had more than 90% similarity to many phenylalanine ammonia-lyase-like proteins in Cucumis sativus L. Therefore, researchers' phylogenetic analysis showed that these candidate genes had the closest evolutionary relationship with Cucumis sativus L. and Cucumis melo L. (Figure 7). Six candidate genes related to SA metabolism were selected to analyze whether they possessed phytohormone cis-regulatory elements. Researchers' results indicated that NPR and WRKY29 possessed an SA responsive element, whereas NPR1, WRKY, WRKY6, WRKY29, and WRKY72 possessed MeJA- and ABA-responsive elements (Table 1).
| Gene ID | Gene Name | Cis-Acting Element |
Function | Sequence | Number |
|---|---|---|---|---|---|
| Cla002899 | NPR1 | ABRE | ABA | ACGTG | 1 |
| Cla019154 | NPR | CGTCA-motif | MeJA | CGTCA | 2 |
| TCA-element | SA | CCATCTTTTT, TCAGAAGAGG |
5 | ||
| Cla022362 | WRKY | ABRE | ABA | AACCCGG | 1 |
| CGTCA-motif | MeJA | CGTCA | 1 | ||
| Cla002084 | WRKY6 | CGTCA-motif | MeJA | CGTCA | 1 |
| Cla005515 | WRKY2 | ABRE | ABA | CACGTG, ACGTG | 5 |
| CGTCA-motif | MeJA | CGTCA | 2 | ||
| TCA-element | SA | CCATCTTTTT | 1 | ||
| Cla010867 | WRKY72 | ABRE | ABA | ACGTG | 2 |
| CGTCA-motif | MeJA | CGTCA | 3 |
This entry is adapted from the peer-reviewed paper 10.3390/plants11030293
References
- Everts, K.L.; Himmelstein, J.C. Fusarium wilt of watermelon: Towards sustainable management of a re-emerging plant disease. Crop Prot. 2015, 73, 93–99.
- Li, C.X.; Fu, X.P.; Zhou, X.G.; Liu, S.W.; Xia, Y.; Li, N.H.; Zhang, X.X.; Wu, F.Z. Treatment with Wheat Root Exudates and Soil Microorganisms from Wheat/Watermelon Companion Cropping Can Induce Watermelon Disease Resistance Against Fusarium oxysporum f. sp. niveum. Plant Dis. 2019, 103, 1693–1702.
- Zhu, F.; Xiao, J.; Zhang, Y.; Wei, L.; Liang, Z. Dazomet application suppressed watermelon wilt by the altered soil microbial community. Sci. Rep. 2020, 10, 21668.
- Kostyn, K.M.; Czemplik, A.; Kulma, M.; Bortniczuk, J.; Skala, J.; Szopa., J. Genes of phenylpropanoid pathway are activated in early response to fusarium attack in flax plants. Plant Sci. 2012, 190, 103–115.
- Everts, K.; Egel, D.S.; Langston, D.; Zhou, X.G. Chemical management of Fusarium wilt of watermelon. Crop Prot. 2014, 66, 114–119.
- Zhu, F.Y.; Tian, C.; Zhang, Y.; Xiao, J.L.; Wei, L.; Liang, Z.H. Effects of different fertilization treatments on soil microbial community structure and the occurrence of watermelon wilt. Chin. J. Biol. Control 2018, 34, 589–597. (In Chinese)
- Zhu, F.Y.; Zhang, Y.; Xiao, J.L.; Wei, L.; Liang, Z.H. Regulation of soil microbial community structures and watermelon Fusarium wilt by using bio-organic fertilizer. Acta Microbiol. Sin. 2019, 59, 2323–2333. (In Chinese)
- Ren, L.; Huo, H.; Zhang, F.; Hao, W.; Xiao, L.; Dong, C.; Xu, G. The components of rice and watermelon root exudates and their effects on pathogenic fungus and watermelon defense. Plant Signal. Behav. 2016, 11, e1187357.
- Moreno-Velandia, C.A.; Izquierdo-García, L.F.; Ongena, M.; Kloepper, J.W.; Cotes, A.M. Soil sterilization, pathogen and antagonist concentration affect biological control of fusarium wilt of cape gooseberry by bacillus velezensis bs006. Plant Soil 2018, 435, 39–55.
- Pandey, A.; Khan, M.K.; Isik, R.; Turkmen, O.; Acar, R.; Seymen, M.; Hakki, E.E. Genetic diversity and population structure of watermelon (Citrullus sp.) genotypes. 3 Biotech 2019, 9, 210.
- Lv, H.; Cao, H.; Nawaz, M.A.; Sohail, H.; Huang, Y.; Cheng, F.; Kong, Q.; Bie, Z. Wheat Intercropping Enhances the Resistance of Watermelon to Fusarium Wilt. Front. Plant Sci. 2018, 9, 696.
- Xu, W.; Wang, Z.; Wu, F. Companion cropping with wheat increases resistance to Fusarium wilt in watermelon and the roles of root exudates in watermelon root growth. Physiol. Mol. Plant Pathol. 2015, 90, 12–20.
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36.
- Radojicic, A.; Li, X.; Zhang, Y. Salicylic acid: A double-edged sword for programed cell death in plants. Front. Plant Sci. 2018, 9, 1133.
- Wildermuth, C.M.; Dewdney, J.; Wu, G.; Ausubel, M.F. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565.
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467.e15.
- Metraux, J.P.; Signer, H.; Ryals, J.; Ward, E.; Wyss-Benz, M.; Gaudin, J.; Raschdorf, K.; Schmid, E.; Blum, W.; Inverardi, B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 1990, 250, 1004–1006.
- Mandal, S.; Mallick, N.; Mitra, A. Salicylic acid-induced resistance to fusarium oxysporum f. Sp. Lycopersici in tomato. Plant Physiol. Biochem. 2009, 47, 642–649.
- Dou, T.; Shao, X.; Hu, C.; Liu, S.; Sheng, O.; Bi, F.; Deng, G.; Ding, L.; Li, C.; Dong, T.; et al. Host-induced gene silencing of foc tr4 erg6/11 genes exhibits superior resistance to fusarium wilt of banana. Plant Biotechnol. J. 2020, 18, 11–13.
- Li, C.; Liu, Z.; Zhang, Q.; Wang, R.; Xiao, L.; Ma, H.; Chong, K.; Xu, Y. Skp1 is involved in abscisic acid signalling to regulate seed germination, stomatal opening and root growth in arabidopsis thaliana. Plant Cell Environ. 2012, 35, 952–965.
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863.
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.E.; Ali, H.M.; Elshikh, M.S. Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front. Physiol. 2017, 8, 716.
- Malamy, J.P.; Carr, P.J.; Klessig, F.D.; Raskin, I. Salicylic acid a likely endogenous signal in the resistance response of tobacco to viral infection. Science 1990, 250, 1002–1004.
- Wang, Z.; Jia, C.; Li, J.; Huang, S.; Xu, B.; Jin, Z. Activation of salicylic acid metabolism and signal transduction can enhance resistance to fusarium wilt in banana (Musa acuminata L. AAA group, cv. Cavendish). Funct. Integr. Genom. 2015, 15, 47–62.
- Zhu, F.Y.; Wang, Z.W.; Fang, Y.; Tong, J.H.; Xiang, J.; Yang, K.K.; Wang, R.Z. Study on the role of phytohormones in resistance to watermelon fusarium wilt. Plants 2022, 11, 156.
- Xiao, J.L.; Zhang, Y.; Li, J.G.; Zhu, F.Y.; Wei, L.; Liang, Z.H. Establishment of real-time pcr system for quantitatively detecting fusarium oxysporum f. Sp. Niveum in soil. J. Plant Prot. 2018, 45, 921–922.
