Diabetes mellitus is a comprehensive expression to identify a condition of chronic hyperglycemia whose causes derive from different metabolic disorders characterized by altered insulin secretion or faulty insulin effect on its targets or often both mechanisms . Atherosclerosis (ATS) is the most frequent cause of arterial vasculopathy and is undoubtedly an insidious condition: it is unlikely to be the trigger in coronary artery disease, ischemic stroke, and peripheral artery disease (PAD) on its own; instead, it acts together with other chronic degenerative diseases such as arterial hypertension and diabetes mellitus to constitute the physiopathological basis of cardio- and cerebrovascular accidents.
- diabetes
- stroke
- cerebrovascular disease
- atherosclerosis
Vascular Complications of Diabetes Mellitus
1. Vascular Complications of Diabetes Mellitus
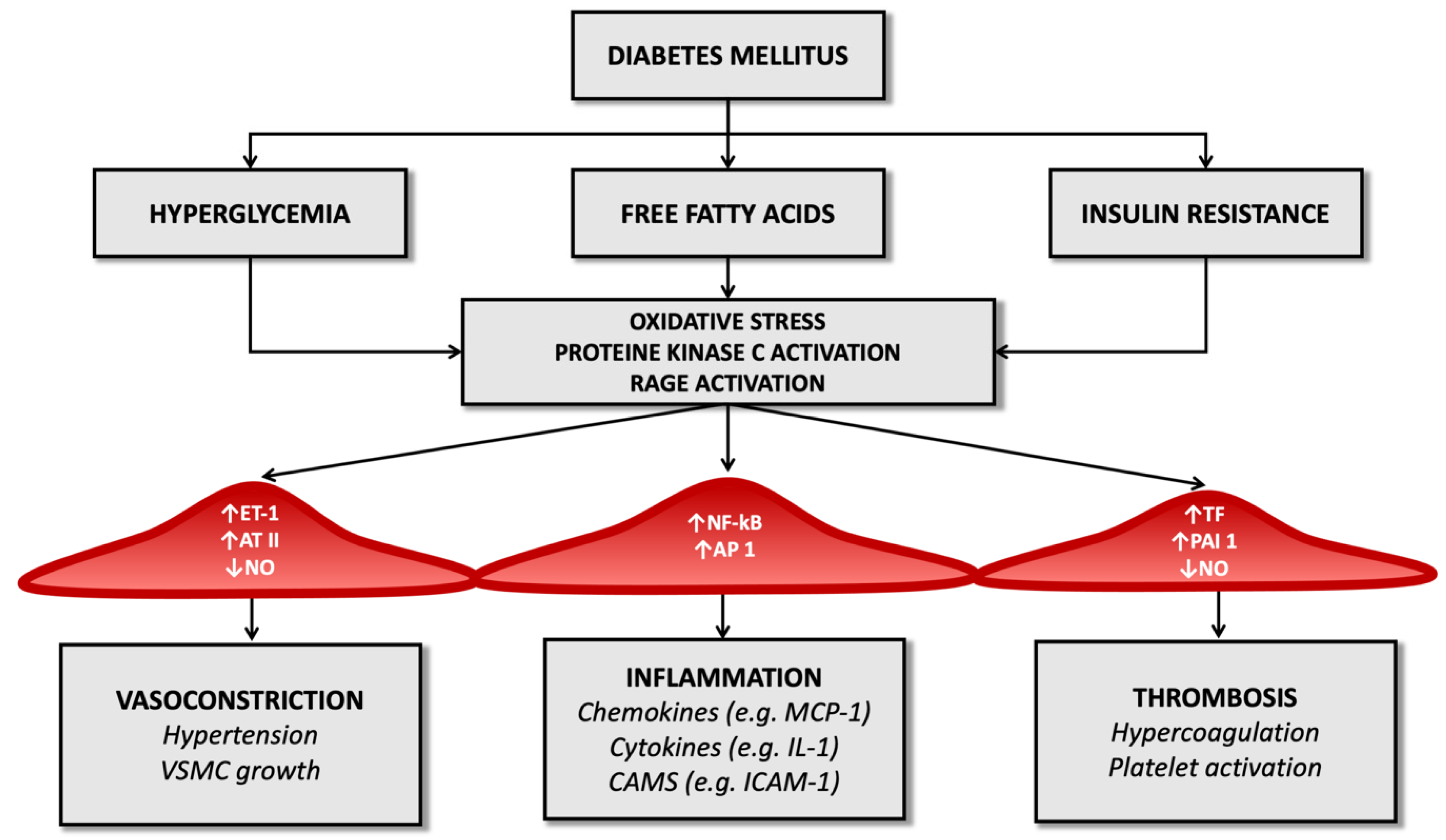
2. Molecular Pathology of Vascular Damage in Diabetes Mellitus and Atherosclerosis
3. Epidemiology of Ischemic Stroke in T2DM Patients
This entry is adapted from the peer-reviewed paper 10.3390/ijms23042397
References
- Heyden, S.; Gerber, C.J. Atherosclerotic Cerebrovascular Disease —Its Nature and Management. Am. J. Med. 1969, 46, 763–773.
- Królewski, A.S.; Czyzyk, A.; Janeczko, D.; Kopczyński, J. Mortality from Cardiovascular Diseases among Diabetics. Diabetologia 1977, 13, 345–350.
- Abbott, R.D.; Donahue, R.P.; MacMahon, S.W.; Reed, D.M.; Yano, K. Diabetes and the Risk of Stroke. JAMA 1987, 257, 949–952.
- Stamler, J.; Vaccaro, O.; Neaton, J.D.; Wentworth, D. Diabetes, Other Risk Factors, and 12-Yr Cardiovascular Mortality for Men Screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993, 16, 434–444.
- You, R.X.; McNeil, J.J.; O’Malley, H.M.; Davis, S.M.; Thrift, A.G.; Donnan, G.A. Risk Factors for Stroke Due to Cerebral Infarction in Young Adults. Stroke 1997, 28, 1913–1918.
- Rohr, J.; Kittner, S.; Feeser, B.; Hebel, J.R.; Whyte, M.G.; Weinstein, A.; Kanarak, N.; Buchholz, D.; Earley, C.; Johnson, C.; et al. Traditional Risk Factors and Ischemic Stroke in Young Adults: The Baltimore-Washington Cooperative Young Stroke Study. Arch. Neurol. 1996, 53, 603–607.
- Hill, M.D. Stroke and Diabetes Mellitus. Handb. Clin. Neurol. 2014, 126, 167–174.
- Folsom, A.R.; Rasmussen, M.L.; Chambless, L.E.; Howard, G.; Cooper, L.S.; Schmidt, M.I.; Heiss, G. Prospective Associations of Fasting Insulin, Body Fat Distribution, and Diabetes with Risk of Ischemic Stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Diabetes Care 1999, 22, 1077–1083.
- Goldstein, L.B.; Adams, R.; Becker, K.; Furberg, C.D.; Gorelick, P.B.; Hademenos, G.; Hill, M.; Howard, G.; Howard, V.J.; Jacobs, B.; et al. Primary Prevention of Ischemic Stroke: A Statement for Healthcare Professionals from the Stroke Council of the American Heart Association. Circulation 2001, 103, 163–182.
- Himmelmann, A.; Hansson, L.; Svensson, A.; Harmsen, P.; Holmgren, C.; Svanborg, A. Predictors of Stroke in the Elderly. Acta Med. Scand. 1988, 224, 439–443.
- Kuusisto, J.; Mykkänen, L.; Pyörälä, K.; Laakso, M. Non-Insulin-Dependent Diabetes and Its Metabolic Control Are Important Predictors of Stroke in Elderly Subjects. Stroke 1994, 25, 1157–1164.
- Verma, S.; Anderson, T.J. The Ten Most Commonly Asked Questions about Endothelial Function in Cardiology. Cardiol. Rev. 2001, 9, 250–252.
- Sarkar, R.; Meinberg, E.G.; Stanley, J.C.; Gordon, D.; Webb, R.C. Nitric Oxide Reversibly Inhibits the Migration of Cultured Vascular Smooth Muscle Cells. Circ. Res. 1996, 78, 225–230.
- Kubes, P.; Suzuki, M.; Granger, D.N. Nitric Oxide: An Endogenous Modulator of Leukocyte Adhesion. Proc. Natl. Acad. Sci. USA 1991, 88, 4651–4655.
- De Vriese, A.S.; Verbeuren, T.J.; Van de Voorde, J.; Lameire, N.H.; Vanhoutte, P.M. Endothelial Dysfunction in Diabetes. Endothel. Dysfunct. Diabetes. Br. J. Pharmacol. 2000, 130, 963–974.
- Milstien, S.; Katusic, Z. Oxidation of Tetrahydrobiopterin by Peroxynitrite: Implications for Vascular Endothelial Function. Biochem. Biophys. Res. Commun. 1999, 263, 681–684.
- Hennes, M.M.; O’Shaughnessy, I.M.; Kelly, T.M.; LaBelle, P.; Egan, B.M.; Kissebah, A.H. Insulin-Resistant Lipolysis in Abdominally Obese Hypertensive Individuals. Hypertension 1996, 28, 120–126.
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High Glucose Level and Free Fatty Acid Stimulate Reactive Oxygen Species Production through Protein Kinase C--Dependent Activation of NAD(P)H Oxidase in Cultured Vascular Cells. Diabetes 2000, 49, 1939–1945.
- Hopfner, R.L.; Gopalakrishnan, V. Endothelin: Emerging Role in Diabetic Vascular Complications. Diabetologia 1999, 42, 1383–1394.
- Quehenberger, P.; Bierhaus, A.; Fasching, P.; Muellner, C.; Klevesath, M.; Hong, M.; Stier, G.; Sattler, M.; Schleicher, E.; Speiser, W.; et al. Endothelin 1 Transcription Is Controlled by Nuclear Factor-KappaB in AGE-Stimulated Cultured Endothelial Cells. Diabetes 2000, 49, 1561–1570.
- Libby, P. Inflammation in Atherosclerosis. Nature 2002, 420, 868–874.
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39.
- Khan, M.I.; Pichna, B.A.; Shi, Y.; Bowes, A.J.; Werstuck, G.H. Evidence Supporting a Role for Endoplasmic Reticulum Stress in the Development of Atherosclerosis in a Hyperglycaemic Mouse Model. Antioxid. Redox Signal. 2009, 11, 2289–2298.
- Schmidt, A.M. Highlighting Diabetes Mellitus: The Epidemic Continues. Arter. Thromb. Vasc. Biol. 2018, 38, e1–e8.
- Rösen, P.; Nawroth, P.P.; King, G.; Möller, W.; Tritschler, H.J.; Packer, L. The Role of Oxidative Stress in the Onset and Progression of Diabetes and Its Complications: A Summary of a Congress Series Sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes/Metab. Res. Rev. 2001, 17, 189–212.
- Zeiher, A.M.; Fisslthaler, B.; Schray-Utz, B.; Busse, R. Nitric Oxide Modulates the Expression of Monocyte Chemoattractant Protein 1 in Cultured Human Endothelial Cells. Circ. Res. 1995, 76, 980–986.
- Christlieb, A.R.; Janka, H.U.; Kraus, B.; Gleason, R.E.; Icasas-Cabral, E.A.; Aiello, L.M.; Cabral, B.V.; Solano, A. Vascular Reactivity to Angiotensin II and to Norepinephrine in Diabetic Subjects. Diabetes 1976, 25, 268–274.
- Nugent, A.G.; McGurk, C.; Hayes, J.R.; Johnston, G.D. Impaired Vasoconstriction to Endothelin 1 in Patients with NIDDM. Diabetes 1996, 45, 105–107.
- Tesfamariam, B.; Cohen, R.A. Enhanced Adrenergic Neurotransmission in Diabetic Rabbit Carotid Artery. Cardiovasc. Res. 1995, 29, 549–554.
- McDaid, E.A.; Monaghan, B.; Parker, A.I.; Hayes, J.R.; Allen, J.A. Peripheral Autonomic Impairment in Patients Newly Diagnosed with Type II Diabetes. Diabetes Care 1994, 17, 1422–1427.
- Fukumoto, H.; Naito, Z.; Asano, G.; Aramaki, T. Immunohistochemical and Morphometric Evaluations of Coronary Atherosclerotic Plaques Associated with Myocardial Infarction and Diabetes Mellitus. J. Atheroscler. Thromb. 1998, 5, 29–35.
- Taguchi, S.; Oinuma, T.; Yamada, T. A Comparative Study of Cultured Smooth Muscle Cell Proliferation and Injury, Utilizing Glycated Low Density Lipoproteins with Slight Oxidation, Auto-Oxidation, or Extensive Oxidation. J. Atheroscler. Thromb. 2000, 7, 132–137.
- Assert, R.; Scherk, G.; Bumbure, A.; Pirags, V.; Schatz, H.; Pfeiffer, A.F. Regulation of Protein Kinase C by Short Term Hyperglycemia in Human Platelets in Vivo and in Vitro. Diabetologia 2001, 44, 188–195.
- Vinik, A.I.; Erbas, T.; Park, T.S.; Nolan, R.; Pittenger, G.L. Platelet Dysfunction in Type 2 Diabetes. Diabetes Care 2001, 24, 1476–1485.
- Carr, M.E. Diabetes Mellitus: A Hypercoagulable State. J. Diabetes Complicat. 2001, 15, 44–54.
- Nordt, T.K.; Bode, C. Impaired Endogenous Fibrinolysis in Diabetes Mellitus: Mechanisms and Therapeutic Approaches. Semin. Thromb. Hemost. 2000, 26, 495–501.
- Joshi, M.B.; Lad, A.; Prasad, A.S.B.; Balakrishnan, A.; Ramachandra, L.; Satyamoorthy, K. High Glucose Modulates IL-6 Mediated Immune Homeostasis through Impeding Neutrophil Extracellular Trap Formation. FEBS Lett. 2013, 587, 2241–2246.
- Ye, Y.; Zeng, Z.; Jin, T.; Zhang, H.; Xiong, X.; Gu, L. The Role of High Mobility Group Box 1 in Ischemic Stroke. Front. Cell. Neurosci. 2019, 13, 127.
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50.
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492.
- Bertoni, A.G.; Krop, J.S.; Anderson, G.F.; Brancati, F.L. Diabetes-Related Morbidity and Mortality in a National Sample of U.S. Elders. Diabetes Care 2002, 25, 471–475.
- Katakura, M.; Naka, M.; Kondo, T.; Nishii, N.; Komatsu, M.; Sato, Y.; Yamauchi, K.; Hiramatsu, K.; Ikeda, M.; Aizawa, T.; et al. Prospective Analysis of Mortality, Morbidity, and Risk Factors in Elderly Diabetic Subjects: Nagano Study. Diabetes Care 2003, 26, 638–644.
- Kannel, W.B.; McGee, D.L. Diabetes and Cardiovascular Disease. The Framingham Study. JAMA 1979, 241, 2035–2038.
- Kissela, B.M.; Khoury, J.; Kleindorfer, D.; Woo, D.; Schneider, A.; Alwell, K.; Miller, R.; Ewing, I.; Moomaw, C.J.; Szaflarski, J.P.; et al. Epidemiology of Ischemic Stroke in Patients with Diabetes: The Greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care 2005, 28, 355–359.
- Almdal, T.; Scharling, H.; Jensen, J.S.; Vestergaard, H. The Independent Effect of Type 2 Diabetes Mellitus on Ischemic Heart Disease, Stroke, and Death: A Population-Based Study of 13,000 Men and Women with 20 Years of Follow-Up. Arch. Intern. Med. 2004, 164, 1422–1426.
- Tuttolomondo, A.; Pinto, A.; Salemi, G.; Di Raimondo, D.; Di Sciacca, R.; Fernandez, P.; Ragonese, P.; Savettieri, G.; Licata, G. Diabetic and Non-Diabetic Subjects with Ischemic Stroke: Differences, Subtype Distribution and Outcome. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 152–157.
- Mulnier, H.E.; Seaman, H.E.; Raleigh, V.S.; Soedamah-Muthu, S.S.; Colhoun, H.M.; Lawrenson, R.A.; De Vries, C.S. Risk of Stroke in People with Type 2 Diabetes in the UK: A Study Using the General Practice Research Database. Diabetologia 2006, 49, 2859–2865.
- Selvin, E.; Coresh, J.; Shahar, E.; Zhang, L.; Steffes, M.; Sharrett, A.R. Glycaemia (Haemoglobin A1c) and Incident Ischaemic Stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol. 2005, 4, 821–826.
- Guerrero-Romero, F.; Rodríguez-Morán, M. Proteinuria Is an Independent Risk Factor for Ischemic Stroke in Non-Insulin-Dependent Diabetes Mellitus. Stroke 1999, 30, 1787–1791.
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Kearney, P.M.; Blackwell, L.; Collins, R.; Keech, A.; Simes, J.; Peto, R.; Armitage, J.; Baigent, C. Efficacy of Cholesterol-Lowering Therapy in 18,686 People with Diabetes in 14 Randomised Trials of Statins: A Meta-Analysis. Lancet 2008, 371, 117–125.
- UK Prospective Diabetes Study Group. Tight Blood Pressure Control and Risk of Macrovascular and Microvascular Complications in Type 2 Diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998, 317, 703–713.
- PROGRESS Collaborative Group Randomised Trial of a Perindopril-Based Blood-Pressure-Lowering Regimen among 6105 Individuals with Previous Stroke or Transient Ischaemic Attack. Lancet 2001, 358, 1033–1041.
- Lichtman, J.H.; Krumholz, H.M.; Wang, Y.; Radford, M.J.; Brass, L.M. Risk and Predictors of Stroke after Myocardial Infarction among the Elderly: Results from the Cooperative Cardiovascular Project. Circulation 2002, 105, 1082–1087.
- D’Ancona, G.; de Ibarra, J.I.S.; Baillot, R.; Mathieu, P.; Doyle, D.; Metras, J.; Desaulniers, D.; Dagenais, F. Determinants of Stroke after Coronary Artery Bypass Grafting. Eur. J. Cardio-Thorac. Surg. 2003, 24, 552–556.
- Anderson, C.S.; Carter, K.N.; Hackett, M.L.; Feigin, V.; Barber, P.A.; Broad, J.B.; Bonita, R.; Auckland Regional Community Stroke (ARCOS) Study Group. Trends in Stroke Incidence in Auckland, New Zealand, during 1981 to 2003. Stroke 2005, 36, 2087–2093.
- Benatru, I.; Rouaud, O.; Durier, J.; Contegal, F.; Couvreur, G.; Bejot, Y.; Osseby, G.V.; Ben Salem, D.; Ricolfi, F.; Moreau, T.; et al. Stable Stroke Incidence Rates but Improved Case-Fatality in Dijon, France, from 1985 to 2004. Stroke 2006, 37, 1674–1679.
- Rothwell, P.M.; Coull, A.J.; Giles, M.F.; Howard, S.C.; Silver, L.E.; Bull, L.M.; Gutnikov, S.A.; Edwards, P.; Mant, D.; Sackley, C.M.; et al. Change in Stroke Incidence, Mortality, Case-Fatality, Severity, and Risk Factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004, 363, 1925–1933.
- Gray, C.S.; Scott, J.F.; French, J.M.; Alberti, K.G.M.M.; O’Connell, J.E. Prevalence and Prediction of Unrecognised Diabetes Mellitus and Impaired Glucose Tolerance Following Acute Stroke. Age Ageing 2004, 33, 71–77.
- Karapanayiotides, T.; Piechowski-Jozwiak, B.; van Melle, G.; Bogousslavsky, J.; Devuyst, G. Stroke Patterns, Etiology, and Prognosis in Patients with Diabetes Mellitus. Neurology 2004, 62, 1558–1562.
- Jackson, C.; Sudlow, C. Are Lacunar Strokes Really Different? A Systematic Review of Differences in Risk Factor Profiles between Lacunar and Nonlacunar Infarcts. Stroke 2005, 36, 891–901.
- Schulz, U.; Rothwell, P. Differences in Vascular Risk Factors Between Etiological Subtypes of Ischemic Stroke. Stroke 2003, 34, 2050–2059.
