Speckle tracking echocardiography is a novel technique to quantify cardiac function and deformation. It has been applied in a series of cardiovascular diseases for the evaluation of early cardiac impairment. Cardiac damage usually occurs earlier in patients with primary aldosteronism than those with primary hypertension, probably because aldosterone hypersecretion is more commonly observed in the former than the latter patients.
- speckle tracking echocardiography
- primary aldosteronism
- cardiac dysfunction
1. Introduction
2. Diagnosis and Screening of Primary Aldosteronism
3. Left Cardiac Evaluation
3.1. Left Ventricular Remodeling
3.2. Systolic Dysfunction
3.3. Diastolic Dysfunction
4. Right Cardiac Evaluation
5. Cardiac Myocardial Work Evaluation
6. Myocardial Fibrosis
7. Treatment Effect on Cardiac Structure and Function
| First Author | Year | Study Design | Participants | No. of Participants | Age (y) | Sex (Male) | Outcome Measures | Results | Summary of Findings |
|---|---|---|---|---|---|---|---|---|---|
| Rossi GP [9] | 1996 | Cross-sectional | PA vs. PH | 34 vs. 34 | 51 ± 13 vs. 49 ± 12 | 18 vs. 18 | LVMI | 112 ± 5 vs. 98 ± 4 g/m2 | Significantly greater LVMI and higher prevalence of LVH in PA than PH |
| Matsumura K [27] | 2006 | Cross-sectional | PA vs. Renovascular hypertension | 25 vs. 29 | 47 ± 2 vs. 45 ± 4 | 13 vs. 10 | LVMI | 154 ± 7 vs. 135 ± 9 g/m2 | Higher prevalence of LVH in PA than renovascular hypertension |
| Muiesan ML [10] | 2008 | Cross-sectional | PA vs. PH | 125 vs. 125 | 50 ± 11 vs. 51 ± 11 | 71 vs. 71 | LVMI | 50 ± 17 vs. 40 ± 11 g/m2.7 | Significantly higher prevalence of inappropriate LVMI in the absence of traditionally defined LVH in PA than PH |
| Yang Y [12] | 2017 | Cross-sectional | PA vs. PH | 100 vs. 100 | 50 ± 12 vs. 50 ± 12 | 58 vs. 58 | LAVI&E/e’ | LAVI: 23 ± 6 vs. 21 ± 6 mL/m2 E/e’: 13.5 ± 4.3 vs. 11.9 ± 3.3 |
Significantly lower e’ and higher E/e’ in PA than PH, in addition to left atrial enlargement |
| Catena C [41] | 2007 | Prospective longitudinal | PA | Surgery 24 vs. Drug treatments 30 | 53 ± 12 in PA patients | 38 inPA patients | LVMI | 53 ± 11 vs. 52 ± 11 g/m2.7 at baseline 45 ± 12 vs. 49 ± 11 g/m2.7 at 1 year 43 ± 11 vs. 44 ± 11 g/m2.7 at the end of study |
Earlier response of LVM regression in surgery than drug treatment but later comparable in the two groups during an average of 6.4 years follow-up |
| Lin YH [29] | 2011 | Prospective longitudinal | PA | Surgery 11 | 47 ± 8 | 5 | LVMI | 153 ± 31 at baseline vs. 116 ± 12 g/m2 at 1 year | Significant regression in LVMI at 1 year |
| Ori Y [104] | 2013 | Retrospective | PA | Drug treatment 48 | 61 ± 10 | 28 | LVMI | 142 ± 28 at baseline, 121 ± 21 at 1 year and 112 ± 24 g/m2 at 3 years | Significant decrease in LVMI at 1 year and normalized at 3 years |
| Rossi GP [106] | 2013 | Prospective longitudinal | PA | Surgery 110 vs. Drug treatment 70 | 51 ± 12 in PA patients | 57 in PA patients | LVMI | 53 ± 13 vs. 50 ± 11 g/m2.7 at baseline 49 ± 10 vs. 47 ± 8 g/m2.7 during follow-up |
Significant regression in LVMI in surgery but with slight decrease in drug treatment at a median of 36 months follow-up |
| Indra T [111] | 2015 | Prospective longitudinal | PA | Surgery 15 vs. Drug treatment 16 | 49 ± 11 vs. 51 ± 7 | 9 vs. 11 | LVMI&E/e’ | LVMI: 50 ± 12 vs. 53 ± 12 g/m2.7 at baseline 39 ± 9 vs. 49 ± 11 g/m2.7 at the end of study E/e’: 9.6 ± 3.0 vs. 14.4 ± 4.4 at baseline 7.1 ± 1.1 vs. 8.7 ± 1.0 at the end of study |
Significant decrease in E/e’ in both surgery and drug treatment groups, with regression of LVMI only in surgery group |
| Freel EM [91] | 2012 | Cross-sectional | PA vs. PH | 27 vs. 53 | 54 ± 11 vs. 55 ± 9 | 21 vs. 42 | LGE | 70% vs. 13% | 4.3 times higher prevalence of non-infarct related replacement fibrosis in PA than PH |
| Su MY [92] | 2012 | Cross-sectional | PA vs. Controls | 25 vs. 12 | 50 ± 13 vs. 49 ± 14 | 6 vs. 7 | EV | 0.43 ± 0.05 vs. 0.36 ± 0.07 | Significantly increased diffuse fibrosis in PA compared with controls |
| First Author | Year | Study Design | Participants | No. of Participants | Age (y) | Sex (Male) | Outcome Measures | Results | Summary of Findings |
|---|---|---|---|---|---|---|---|---|---|
| Chen ZW [34] | 2018 | Cross-sectional | PA vs. PH | 36 vs. 31 | 49 ± 11 vs. 53 ± 12 | 15 vs. 16 | GLS | −17.8 ± 2.4 vs. −20.3 ± 2.3% | Significantly lower GLS in PA than PH |
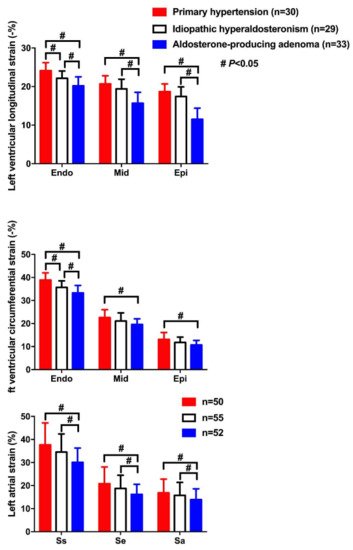
| Wang D [39] | 2019 | Cross-sectional | APA, IHAand PH | 33, 29 and 30 | 49 ± 10, 52 ± 8 and 51 ± 20 | 20, 22 and 18 | LS&CS | LSendo: −20.2 ± 2.3, −22.1 ± 1.9 and −24.1 ± 2.1% LSmid: −15.7 ± 2.8, −19.4 ± 2.5 and −20.7 ± 21% LSepi: −15.8 ± 2.1, −19.6 ± 2.2 and −21.2 ± 1.9% CSendo: −33.3 ± 3.2, −35.7 ± 2.8 and −38.9 ± 3.1% CSmid: −19.6 ± 2.4, −21.1 ± 3.5 and −22.6 ± 3.4% CSepi: −10.7 ± 2.0, −11.8 ± 2.3 and −13.1 ± 3.0% |
Lowest CS and LS in endocardium (endo), midmyocardium (mid) and epicardium (epi) in APA, intermediate in IHA, and highest in PH |
| Wang D [40] | 2019 | Cross-sectional | APA, IHA and PH | 52, 55 and 50 | 52 ± 11, 51 ± 10 and 50 ± 17 | 35, 34 and 33 | LAS&LASR | LASs: 30.1 ± 6.2, 34.5 ± 7.9 and 37.7 ± 9.5% LASe: 16.2 ± 4.4, 18.8 ± 5.7 and 20.8 ± 7.3% LASa: 13.9 ± 4.7, 15.8 ± 5.6 and 16.9 ± 6.0% LASRs: 1.8 ± 0.6, 1.9 ± 0.4 and 2.1 ± 0.7/s LASRe: 1.6 ± 0.4, 1.7 ± 0.4 and 1.9 ± 0.6/s LASRa: 1.5 ± 0.5, 1.6 ± 0.6 and 1.8 ± 0.5/s |
Significantly lower LAS and LASR during atrial reservoir (s), conduit (e) and contractile (a) phases in APA than IHA and PH |
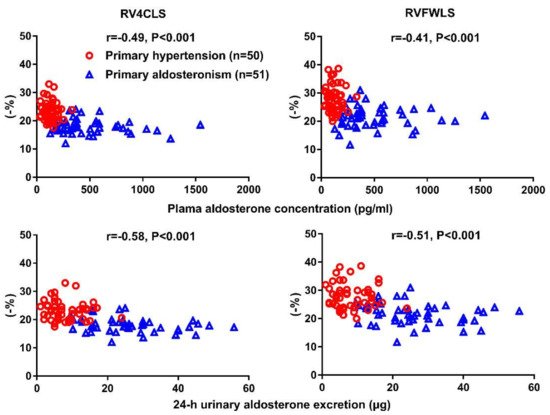
| Chen YL [66] | 2020 | Cross-sectional | PA vs. PH | 51 vs. 50 | 51 ± 11 vs. 53 ± 11 | 34 vs. 30 | RV4CLS & RVFWLS | RV4CLS: −18.1 ± 2.5 vs.−23.3 ± 3.4% RVFWLS: −21.7 ± 3.7 vs. −27.9 ± 4.5% |
Significantdecrease in both RV4CLS and RVFWLS in PA than PH |
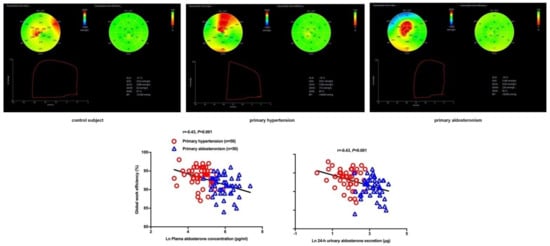
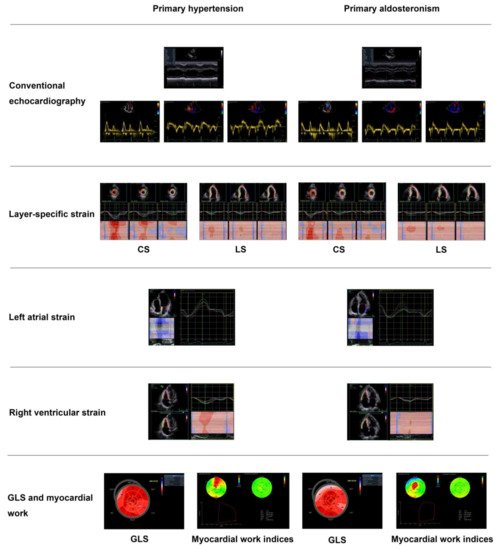
| Chen YL [79] | 2021 | Cross-sectional | PA vs. PH | 50 vs. 50 | 51 ± 10 vs. 55 ± 11 | 32 vs. 33 | Strain (GLS) and myocardial work indices (GWI, GCW, GWW, & GWE) | GLS: −18.0 ± 2.1 vs. −19.2 ± 2.0% GWI: 2336 ± 333 vs. 2366 ± 288 mmHg% GCW: 2494 ± 325 vs. 2524 ± 301 mmHg% GWW: 206 ± 75 vs. 142 ± 56 mmHg% GWE: 91.1 ± 2.7 vs. 93.5 ± 2.5% |
Significant decrease in GLS and GWE and increase in GWW in PA than PH, with similar GWI and GCW in the two groups |
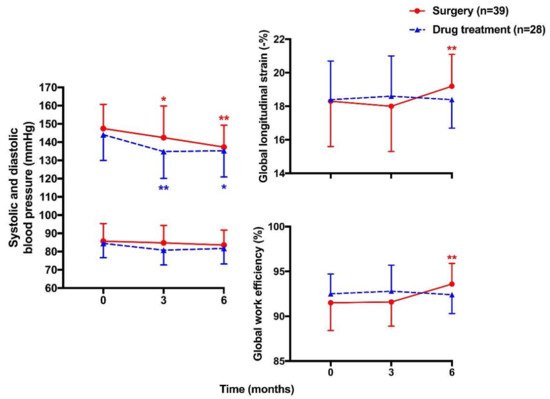
| Chen YL [116] | 2021 | Prospective longitudinal | PA | Surgery 39 vs. Drug treatment 28 | 49 ± 10 vs. 49 ± 12 | 26 vs. 22 | Strain (GLS) and myocardial work indices (GWI, GCW, GWW, & GWE) | GLS: −18.3 ± 2.7 vs. −18.4 ± 2.3% at baseline 19.2 ± 1.9 vs. 18.4 ± 1.7% at 6 months GWI: 2372 ± 388 vs. 2335 ± 341 mmHg% at baseline 2280 ± 344 vs. 2208 ± 306 mmHg% at 6 months GCW: 2510 ± 360 vs. 2437 ± 293 mmHg% at baseline 2436 ± 335 vs. 2330 ± 311 mmHg% at 6 months GWW: 201 ± 87 vs. 164 ± 56 mmHg% at baseline 142 ± 58 vs. 164 ± 53 mmHg% at 6 months GWE: 91.5 ± 3.1 vs. 92.5 ± 2.2% at baseline 93.6 ± 2.3 vs. 92.4 ± 2.1% at 6 months |
Significant improvement in GLS and GWE in surgery but not drug group at 6-month follow-up |
Values are expressed as mean ± SD. APA—aldosterone-producing adenoma; CS—circumferential strain; GCW—global constructive work; GLS—global longitudinal strain; GWE—global work efficiency; GWI—global work index; GWW—global wasted work; IHA—idiopathic hyperaldosteronism; LAS—left atrial strain; LASR—left atrial strain rate; LS—longitudinal strain; PA—primary aldosteronism; PH—primary hypertension; RV4CLS—right ventricular four-chamber longitudinal strain; RVFWLS—right ventricular free wall longitudinal strain.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12020543
References
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056.
- Tadic, M.; Sala, C.; Carugo, S.; Mancia, G.; Grassi, G.; Cuspidi, C. Myocardial strain in hypertension: A meta-analysis of two-dimensional speckle tracking echocardiographic studies. J. Hypertens. 2021, 39, 2103–2112.
- Tadic, M.; Cuspidi, C.; Bombelli, M.; Grassi, G. Right heart remodeling induced by arterial hypertension: Could strain assessment be helpful? J. Clin. Hypertens. 2018, 20, 400–407.
- Kouzu, H.; Yuda, S.; Muranaka, A.; Doi, T.; Yamamoto, H.; Shimoshige, S.; Hase, M.; Hashimoto, A.; Saitoh, S.; Tsuchihashi, K.; et al. Left Ventricular Hypertrophy Causes Different Changes in Longitudinal, Radial, and Circumferential Mechanics in Patients with Hypertension: A Two-Dimensional Speckle Tracking Study. J. Am. Soc. Echocardiogr. 2011, 24, 192–199.
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14.
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314.
- Marwick, T.H.; Gillebert, T.C.; Aurigemma, G.; Chirinos, J.; Derumeaux, G.; Galderisi, M.; Gottdiener, J.; Haluska, B.; Ofili, E.; Segers, P.; et al. Recommendations on the use of echocardiography in adult hypertension: A report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J. Am. Soc. Echocardiogr. 2015, 28, 727–754.
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680.
- Rossi, G.P.; Sacchetto, A.; Visentin, P.; Canali, C.; Graniero, G.R.; Palatini, P.; Pessina, A.C. Changes in Left Ventricular Anatomy and Function in Hypertension and Primary Aldosteronism. Hypertension 1996, 27, 1039–1045.
- Muiesan, M.L.; Salvetti, M.; Paini, A.; Agabiti-Rosei, C.; Monteduro, C.; Galbassini, G.; Belotti, E.; Aggiusti, C.; Rizzoni, D.; Castellano, M.; et al. Inappropriate Left Ventricular Mass in Patients with Primary Aldosteronism. Hypertension 2008, 52, 529–534.
- Tanabe, A.; Naruse, M.; Naruse, K.; Hase, M.; Yoshimoto, T.; Tanaka, M.; Seki, T.; Demura, R.; Demura, H. Left ventricular hypertrophy is more prominent in patients with primary aldosteronism than in patients with other types of secondary hypertension. Hypertens. Res. 1997, 20, 85–90.
- Yang, Y.; Zhu, L.; Xu, J.; Tang, X.; Gao, P. Comparison of left ventricular structure and function in primary aldosteronism and essential hypertension by echocardiography. Hypertens. Res. 2017, 40, 243–250.
- Monticone, S.; Burrello, J.; Tizzani, D.; Bertello, C.; Viola, A.; Buffolo, F.; Gabetti, L.; Mengozzi, G.; Williams, T.A.; Rabbia, F.; et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J. Am. Coll. Cardiol. 2017, 69, 1811–1820.
- Xu, Z.; Yang, J.; Hu, J.; Song, Y.; He, W.; Luo, T.; Cheng, Q.; Ma, L.; Luo, R.; Fuller, P.J.; et al. Primary Aldosteronism in Patients in China With Recently Detected Hypertension. J. Am. Coll. Cardiol. 2020, 75, 1913–1922.
- Douma, S.; Petidis, K.; Doumas, M.; Papaefthimiou, P.; Triantafyllou, A.; Kartali, N.; Papadopoulos, N.; Vogiatzis, K.; Zamboulis, C. Prevalence of primary hyperaldosteronism in resistant hypertension: A retrospective observational study. Lancet 2008, 371, 1921–1926.
- Jansen, P.M.; van den Born, B.-J.H.; Frenkel, W.J.; de Bruijne, E.L.E.; Deinum, J.; Kerstens, M.N.; Smulders, Y.M.; Woittiez, A.J.; Wijbenga, J.A.M.; Zietse, R.; et al. Test characteristics of the aldosterone-to-renin ratio as a screening test for primary aldosteronism. J. Hypertens. 2014, 32, 115–126.
- Hu, Y.; Zhang, J.; Liu, W.; Su, X. Determining the Prevalence of Primary Aldosteronism in Patients With New-Onset Type 2 Diabetes and Hypertension. J. Clin. Endocrinol. Metab. 2020, 105, 1079–1085.
- Chinese Society of Endocrinology. Expert consensus on diagnosis and treatment of primary aldosteronism. Chin. J. Endocrinol. Metab. 2020, 36, 727–736.
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916.
- Mulatero, P.; Monticone, S.; Deinum, J.; Amar, L.; Prejbisz, A.; Zennaro, M.-C.; Beuschlein, F.; Rossi, G.P.; Nishikawa, T.; Morganti, A.; et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: A position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 2020, 38, 1919–1928.
- Chen, S.X.; Du, Y.L.; Zhang, J.; Gong, Y.C.; Hu, Y.R.; Chu, S.L.; He, Q.B.; Song, Y.Y.; Zhu, D.L. Aldosterone-to-renin ratio threshold for screening primary aldosteronism in Chinese hypertensive patients. ZhongHua Xin Xue Guan Bing Za Zhi 2006, 34, 868–872.
- Mulatero, P.; Sechi, L.A.; Williams, T.A.; Lenders, J.W.M.; Reincke, M.; Satoh, F.; Januszewicz, A.; Naruse, M.; Doumas, M.; Veglio, F.; et al. Subtype diagnosis, treatment, complications and outcomes of primary aldosteronism and future direction of research: A position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 2020, 38, 1929–1936.
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21.
- Rossi, G.P.; Sacchetto, A.; Pavan, E.; Palatini, P.; Graniero, G.R.; Canali, C.; Pessina, A.C. Remodeling of the Left Ventricle in Primary Aldosteronism Due to Conn’s Adenoma. Circulation 1997, 95, 1471–1478.
- Pimenta, E.; Gordon, R.D.; Ahmed, A.H.; Cowley, D.; Leano, R.; Marwick, T.H.; Stowasser, M. Cardiac Dimensions Are Largely Determined by Dietary Salt in Patients with Primary Aldosteronism: Results of a Case-Control Study. J. Clin. Endocrinol. Metab. 2011, 96, 2813–2820.
- Palmieri, V.; Wachtell, K.; Gerdts, E.; Bella, J.N.; Papademetriou, V.; Tuxen, C.; Nieminen, M.S.; Dahlöf, B.; de Simone, G.; Devereux, R.B. Left ventricular function and hemodynamic features of inappropriate left ventricular hypertrophy in patients with systemic hypertension: The LIFE Study. Am. Heart J. 2001, 141, 784–791.
- Matsumura, K.; Fujii, K.; Oniki, H.; Oka, M.; Iida, M. Role of Aldosterone in Left Ventricular Hypertrophy in Hypertension. Am. J. Hypertens. 2006, 19, 13–18.
- Tsai, C.-H.; Pan, C.-T.; Chang, Y.-Y.; Chen, Z.-W.; Wu, V.-C.; Hung, C.-S.; Lin, Y.-H. Left ventricular remodeling and dysfunction in primary aldosteronism. J. Hum. Hypertens. 2021, 35, 131–147.
- Lin, Y.-H.; Lee, H.-H.; Liu, K.-L.; Lee, J.-K.; Shih, S.-R.; Chueh, S.-C.; Lin, W.-C.; Lin, L.-C.; Lin, L.-Y.; Chung, S.-D.; et al. Reversal of myocardial fibrosis in patients with unilateral hyperaldosteronism receiving adrenalectomy. Surgery 2011, 150, 526–533.
- Lin, Y.-H.; Wu, X.-M.; Lee, H.-H.; Lee, J.-K.; Liu, Y.-C.; Chang, H.-W.; Lin, C.-Y.; Wu, V.-C.; Chueh, S.-C.; Lin, L.-C.; et al. Adrenalectomy reverses myocardial fibrosis in patients with primary aldosteronism: J. Hypertens. 2012, 30, 1606–1613.
- Rossi, G.P.; Di Bello, V.; Ganzaroli, C.; Sacchetto, A.; Cesari, M.; Bertini, A.; Giorgi, D.; Scognamiglio, R.; Mariani, M.; Pessina, A.C. Excess Aldosterone Is Associated with Alterations of Myocardial Texture in Primary Aldosteronism. Hypertension 2002, 40, 23–27.
- Cesari, M.; Letizia, C.; Angeli, P.; Sciomer, S.; Rosi, S.; Rossi, G.P. Cardiac remodeling in patients with primary and secondary aldosteronism: A tissue Doppler study. Circ. Cardiovasc. Imaging 2016, 9, e004815.
- Finch-Johnston, A.; Gussak, H.; Mobley, J.; Holland, M.; Petrovic, O.; Pérez, J.; Miller, J. Cyclic variation of integrated backscatter: Dependence of time delay on the echocardiographic view used and the myocardial segment analyzed. J. Am. Soc. Echocardiogr. 2000, 13, 9–17.
- Chen, Z.-W.; Huang, K.-C.; Lee, J.-K.; Lin, L.-C.; Chen, C.-W.; Chang, Y.-Y.; Liao, C.-W.; Wu, V.-C.; Hung, C.-S.; Lin, Y.-H. Aldosterone induces left ventricular subclinical systolic dysfunction: A strain imaging study. J. Hypertens. 2018, 36, 353–360.
- Tarascio, M.; Leo, L.A.; Klersy, C.; Murzilli, R.; Moccetti, T.; Faletra, F.F. Speckle-Tracking Layer-Specific Analysis of Myocardial Deformation and Evaluation of Scar Transmurality in Chronic Ischemic Heart Disease. J. Am. Soc. Echocardiogr. 2017, 30, 667–675.
- Ünlü, S.; Mirea, O.; Duchenne, J.; Pagourelias, E.D.; Bézy, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.-U. Comparison of Feasibility, Accuracy, and Reproducibility of Layer-Specific Global Longitudinal Strain Measurements Among Five Different Vendors: A Report from the EACVI-ASE Strain Standardization Task Force. J. Am. Soc. Echocardiogr. 2018, 31, 374–380.e1.
- Kim, D.; Shim, C.Y.; Hong, G.-R.; Park, S.; Cho, I.; Chang, H.-J.; Ha, J.-W.; Chung, N. Differences in left ventricular functional adaptation to arterial stiffness and neurohormonal activation in patients with hypertension: A study with two-dimensional layer-specific speckle tracking echocardiography. Clin. Hypertens. 2017, 23, 21.
- Lee, W.-H.; Liu, Y.-W.; Yang, L.-T.; Tsai, W.-C. Prognostic value of longitudinal strain of subepicardial myocardium in patients with hypertension. J. Hypertens. 2016, 34, 1195–1200.
- Wang, D.; Xu, J.-Z.; Chen, X.; Chen, Y.; Shao, S.; Zhang, W.; Zhu, L.-M.; Xu, T.-Y.; Li, Y.; Wang, J.-G. Speckle-Tracking Echocardiographic Layer-Specific Strain Analysis on Subclinical Left Ventricular Dysfunction in Patients With Primary Aldosteronism. Am. J. Hypertens. 2019, 32, 155–162.
- Wang, D.; Xu, J.-Z.; Chen, X.; Xu, T.-Y.; Zhang, W.; Li, Y.; Wang, Y.G. Left atrial myocardial dysfunction in patients with primary aldosteronism as assessed by speckle-tracking echocardiography. J. Hypertens. 2019, 37, 2032–2040.
- Catena, C.; Colussi, G.; Lapenna, R.; Nadalini, E.; Chiuch, A.; Gianfagna, P.; Sechi, L.A. Long-Term Cardiac Effects of Adrenalectomy or Mineralocorticoid Antagonists in Patients With Primary Aldosteronism. Hypertension 2007, 50, 911–918.
- Chang, Y.-Y.; Lee, H.-H.; Hung, C.-S.; Wu, X.-M.; Lee, J.-K.; Wang, S.-M.; Liao, M.-T.; Chen, Y.-H.; Wu, V.-C.; Wu, K.-D.; et al. Association between urine aldosterone and diastolic function in patients with primary aldosteronism and essential hypertension. Clin. Biochem. 2014, 47, 1329–1332.
- Morris, D.A.; Takeuchi, M.; Krisper, M.; Kohncke, C.; Bekfani, T.; Carstensen, T.; Hassfeld, S.; Dorenkamp, M.; Otani, K.; Takigiku, K.; et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: Multicentre study. Eur. Heart J.—Cardiovasc. Imaging 2015, 16, 364–372.
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415.
- Mondillo, S.; Cameli, M.; Caputo, M.L.; Lisi, M.; Palmerini, E.; Padeletti, M.; Ballo, P. Early Detection of Left Atrial Strain Abnormalities by Speckle-Tracking in Hypertensive and Diabetic Patients with Normal Left Atrial Size. J. Am. Soc. Echocardiogr. 2011, 24, 898–908.
- Xu, T.-Y.; Sun, J.P.; Lee, A.P.-W.; Yang, X.S.; Ji, L.; Zhang, Z.; Li, Y.; Yu, C.-M.; Wang, J.-G. Left Atrial Function as Assessed by Speckle-Tracking Echocardiography in Hypertension. Medicine 2015, 94, e526.
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J.—Cardiovasc. Imaging 2018, 19, 591–600.
- Mandoli, G.E.; Sisti, N.; Mondillo, S.; Cameli, M. Left atrial strain in left ventricular diastolic dysfunction: Have we finally found the missing piece of the puzzle? Heart Fail. Rev. 2020, 25, 409–417.
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; García-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, Ø.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70.
- Smiseth, O.A.; Morris, D.A.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, F.A.; Galderisi, M.; Gerber, B.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 1–28.
- Burke, M.A.; Katz, D.H.; Beussink, L.; Selvaraj, S.; Gupta, D.K.; Fox, J.; Chakrabarti, S.; Sauer, A.J.; Rich, J.D.; Freed, B.H.; et al. Prognostic Importance of Pathophysiologic Markers in Patients With Heart Failure and Preserved Ejection Fraction. Circ. Heart Fail. 2014, 7, 288–299.
- Nochioka, K.; Querejeta Roca, G.; Claggett, B.; Biering-Sørensen, T.; Matsushita, K.; Hung, C.-L.; Solomon, S.D.; Kitzman, D.; Shah, A.M. Right Ventricular Function, Right Ventricular–Pulmonary Artery Coupling, and Heart Failure Risk in 4 US Communities: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2018, 3, 939–948.
- Oketona, O.; Balogun, M.; Akintomide, A.; Ajayi, O.; Adebayo, R.; Mene-Afejuku, T.; Oketona, O.; Bamikole, O. Right ventricular systolic function in hypertensive heart failure. Vasc. Health Risk Manag. 2017, 13, 353–360.
- Cuspidi, C.; Negri, F.; Giudici, V.; Valerio, C.; Meani, S.; Sala, C.; Esposito, A.; Masaidi, M.; Zanchetti, A.; Mancia, G. Prevalence and clinical correlates of right ventricular hypertrophy in essential hypertension. J. Hypertens. 2009, 27, 854–860.
- Tumuklu, M.M.; Erkorkmaz, U.; Ocal, A. The impact of hypertension and hypertension-related left ventricle hypertrophy on right ventricle function. Echocardiography 2007, 24, 374–384.
- Vriz, O.; Argiento, P.; D’Alto, M.; Ferrara, F.; Vanderpool, R.; Naeije, R.; Bossone, E. Increased pulmonary vascular resistance in early stage systemic hypertension: A resting and exercise stress echocardiography study. Can. J. Cardiol. 2015, 31, 537–543.
- Gregori, M.; Giammarioli, B.; Tocci, G.; Befani, A.; Ciavarella, G.M.; Ferrucci, A.; Paneni, F. Synergic effects of renin and aldosterone on right ventricular function in hypertension: A tissue Doppler study. J. Cardiovasc. Med. 2015, 16, 831–838.
- Gregori, M.; Tocci, G.; Giammarioli, B.; Befani, A.; Ciavarella, G.M.; Ferrucci, A.; Paneni, F. Abnormal Regulation of Renin Angiotensin Aldosterone System Is Associated with Right Ventricular Dysfunction in Hypertension. Can. J. Cardiol. 2014, 30, 188–194.
- Guazzi, M.; Borlaug, B.A. Pulmonary hypertension due to left heart disease. Circulation 2012, 126, 975–990.
- Nakagawa, A.; Yasumura, Y.; Yoshida, C.; Okumura, T.; Tateishi, J.; Yoshida, J.; Abe, H.; Tamaki, S.; Yano, M.; Hayashi, T.; et al. Prognostic importance of right ventricular-vascular uncoupling in acute decompensated heart failure with preserved ejection fraction. Circ. Cardiovasc. Imaging 2020, 13, e011430.
- Vriz, O.; Pirisi, M.; Bossone, E.; Fadl ElMula, F.E.M.; Palatini, P.; Naeije, R. Right ventricular–pulmonary arterial uncoupling in mild-to-moderate systemic hypertension. J. Hypertens. 2020, 38, 274–281.
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713.
- Yoshifuku, S.; Otsuji, Y.; Takasaki, K.; Yuge, K.; Kisanuki, A.; Toyonaga, K.; Lee, S.; Murayama, T.; Nakashima, H.; Kumanohoso, T.; et al. Pseudonormalized doppler totalejection isovolume (Tei) index in patients with right ventricularacute myocardial infarction. Am. J. Cardiol. 2003, 91, 527–531.
- Tadic, M.; Cuspidi, C.; Celic, V.; Pencic-Popovic, B.; Mancia, G. Nocturnal hypertension and right heart remodeling. J. Hypertens. 2018, 36, 136–142.
- Tadic, M.; Cuspidi, C.; Pencic, B.; Ivanovic, B.; Scepanovic, R.; Marjanovic, T.; Jozika, L.; Celic, V. Circadian blood pressure pattern and right ventricular and right atrial mechanics: A two- and three-dimensional echocardiographic study. J. Am. Soc. Hypertens. 2014, 8, 45–53.
- Chen, Y.-L.; Xu, T.-Y.; Xu, J.-Z.; Zhu, L.-M.; Li, Y.; Wang, J.-G. A speckle tracking echocardiographic study on right ventricular function in primary aldosteronism. J. Hypertens. 2020, 38, 2261–2269.
- Van der Bijl, P.; Kostyukevich, M.; El Mahdiui, M.; Hansen, G.; Samset, E.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. A Roadmap to Assess Myocardial Work: From theory to clinical practice. JACC Cardiovasc. Imaging 2019, 12, 2549–2554.
- Boe, E.; Skulstad, H.; Smiseth, O.A. Myocardial work by echocardiography: A novel method ready for clinical testing. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 18–20.
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure–strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733.
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, T.; Smiseth, O.A. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H996–H1003.
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 582–590.
- Galli, E.; Leclercq, C.; Fournet, M.; Hubert, A.; Bernard, A.; Smiseth, O.A.; Mabo, P.; Samset, E.; Hernandez, A.; Donal, E. Value of Myocardial Work Estimation in the Prediction of Response to Cardiac Resynchronization Therapy. J. Am. Soc. Echocardiogr. 2018, 31, 220–230.
- Cauwenberghs, N.; Tabassian, M.; Thijs, L.; Yang, W.-Y.; Wei, F.-F.; Claus, P.; D’hooge, J.; Staessen, J.A.; Kuznetsova, T. Area of the pressure-strain loop during ejection as non-invasive index of left ventricular performance: A population study. Cardiovasc. Ultrasound 2019, 17, 15.
- Edwards, N.F.A.; Scalia, G.M.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Khandheria, B.K.; Chan, J. Global Myocardial Work Is Superior to Global Longitudinal Strain to Predict Significant Coronary Artery Disease in Patients With Normal Left Ventricular Function and Wall Motion. J. Am. Soc. Echocardiogr. 2019, 32, 947–957.
- van der Bijl, P.; Vo, N.M.; Kostyukevich, M.V.; Mertens, B.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Prognostic implications of global, left ventricular myocardial work efficiency before cardiac resynchronization therapy. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 1388–1394.
- Chan, J.; Edwards, N.F.A.; Khandheria, B.K.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Scalia, G.M. A new approach to assess myocardial work by non-invasive left ventricular pressure–strain relations in hypertension and dilated cardiomyopathy. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 31–39.
- Jaglan, A.; Roemer, S.; Perez Moreno, A.C.; Khandheria, B.K. Myocardial work in Stage 1 and 2 hypertensive patients. Eur. Heart J.—Cardiovasc. Imaging 2021, 22, 744–750.
- Sahiti, F.; Morbach, C.; Cejka, V.; Tiffe, T.; Wagner, M.; Eichner, F.A.; Gelbrich, G.; Heuschmann, P.U.; Störk, S. Impact of cardiovascular risk factors on myocardial work—insights from the STAAB cohort study. J. Hum. Hypertens. 2021.
- Chen, Y.-L.; Xu, T.-Y.; Xu, J.-Z.; Zhu, L.-M.; Li, Y.; Wang, J.-G. A non-invasive left ventricular pressure-strain loop study on myocardial work in primary aldosteronism. Hypertens. Res. 2021, 44, 1462–1470.
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469.
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A.C. Assessment of Myocardial Fibrosis with Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903.
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76.
- Ambale-Venkatesh, B.; Lima, J.A.C. Cardiac MRI: A central prognostic tool in myocardial fibrosis. Nat. Rev. Cardiol. 2015, 12, 18–29.
- Lembo, M.; Manzi, M.V.; Mancusi, C.; Morisco, C.; Rao, M.A.E.; Cuocolo, A.; Izzo, R.; Trimarco, B. Advanced imaging tools for evaluating cardiac morphological and functional impairment in hypertensive disease. J. Hypertens. 2022, 40, 4–14.
- Weber, K.T.; Brilla, C.G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991, 83, 1849–1865.
- Rudolph, A.; Abdel-Aty, H.; Bohl, S.; Boyé, P.; Zagrosek, A.; Dietz, R.; Schulz-Menger, J. Noninvasive Detection of Fibrosis Applying Contrast-Enhanced Cardiac Magnetic Resonance in Different Forms of Left Ventricular Hypertrophy. J. Am. Coll. Cardiol. 2009, 53, 284–291.
- Weber, K.T.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Gerling, I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013, 10, 15–26.
- Scully, P.R.; Bastarrika, G.; Moon, J.C.; Treibel, T.A. Myocardial Extracellular Volume Quantification by Cardiovascular Magnetic Resonance and Computed Tomography. Curr. Cardiol. Rep. 2018, 20, 15.
- Kuruvilla, S.; Janardhanan, R.; Antkowiak, P.; Keeley, E.C.; Adenaw, N.; Brooks, J.; Epstein, F.H.; Kramer, C.M.; Salerno, M. Increased Extracellular Volume and Altered Mechanics Are Associated with LVH in Hypertensive Heart Disease, Not Hypertension Alone. JACC Cardiovasc. Imaging 2015, 8, 172–180.
- Pan, J.A.; Michaëlsson, E.; Shaw, P.W.; Kuruvilla, S.; Kramer, C.M.; Gan, L.-M.; Keeley, E.C.; Salerno, M. Extracellular volume by cardiac magnetic resonance is associated with biomarkers of inflammation in hypertensive heart disease. J. Hypertens. 2019, 37, 65–72.
- Freel, E.M.; Mark, P.B.; Weir, R.A.P.; McQuarrie, E.P.; Allan, K.; Dargie, H.J.; McClure, J.D.; Jardine, A.G.; Davies, E.; Connell, J.M.C. Demonstration of Blood Pressure-Independent Noninfarct Myocardial Fibrosis in Primary Aldosteronism: A Cardiac Magnetic Resonance Imaging Study. Circ. Cardiovasc. Imaging 2012, 5, 740–747.
- Su, M.-Y.M.; Wu, V.-C.; Yu, H.-Y.; Lin, Y.-H.; Kuo, C.-C.; Liu, K.-L.; Wang, S.-M.; Chueh, S.-C.; Lin, L.-Y.; Wu, K.-D.; et al. Contrast-enhanced MRI index of diffuse myocardial fibrosis is increased in primary aldosteronism. J. Magn. Reson. Imaging 2012, 35, 1349–1355.
- Redheuil, A.; Blanchard, A.; Pereira, H.; Raissouni, Z.; Lorthioir, A.; Soulat, G.; Vargas-Poussou, R.; Amar, L.; Paul, J.-L.; Helley, D.; et al. Aldosterone-Related Myocardial Extracellular Matrix Expansion in Hypertension in Humans. JACC Cardiovasc. Imaging 2020, 13, 2149–2159.
- Grytaas, M.A.; Sellevåg, K.; Thordarson, H.B.; Husebye, E.S.; Løvås, K.; Larsen, T.H. Cardiac magnetic resonance imaging of myocardial mass and fibrosis in primary aldosteronism. Endocr. Connect. 2018, 7, 413–424.
- Cimino, S.; Canali, E.; Petronilli, V.; Cicogna, F.; De Luca, L.; Francone, M.; Sardella, G.; Iacoboni, C.; Agati, L. Global and regional longitudinal strain assessed by two-dimensional speckle tracking echocardiography identifies early myocardial dysfunction and transmural extent of myocardial scar in patients with acute ST elevation myocardial infarction and relatively preserved LV function. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 805–811.
- Zhao, H.; Lee, A.P.-W.; Li, Z.; Qiao, Z.; Fan, Y.; An, D.; Xu, J.; Pu, J.; Shen, X.; Ge, H.; et al. Impact of Intramyocardial Hemorrhage and Microvascular Obstruction on Cardiac Mechanics in Reperfusion Injury: A Speckle-Tracking Echocardiographic Study. J. Am. Soc. Echocardiogr. 2016, 29, 973–982.
- Wang, J.; Zhang, Y.; Zhang, L.; Tian, F.; Wang, B.; Xie, Y.; Sun, W.; Sun, Z.; Yang, Y.; Lv, Q.; et al. Assessment of myocardial fibrosis using two-dimensional and three-dimensional speckle tracking echocardiography in dilated cardiomyopathy with advanced heart failure. J. Card. Fail. 2021, 27, 651–661.
- Pagourelias, E.D.; Mirea, O.; Duchenne, J.; Unlu, S.; Van Cleemput, J.; Papadopoulos, C.E.; Bogaert, J.; Vassilikos, V.P.; Voigt, J.-U. Speckle tracking deformation imaging to detect regional fibrosis in hypertrophic cardiomyopathy: A comparison between 2D and 3D echo modalities. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1262–1272.
- Mandoli, G.E.; D’Ascenzi, F.; Vinco, G.; Benfari, G.; Ricci, F.; Focardi, M.; Cavigli, L.; Pastore, M.C.; Sisti, N.; De Vivo, O.; et al. Novel approaches in cardiac imaging for non-invasive assessment of left heart myocardial fibrosis. Front. Cardiovasc. Med. 2021, 8, 614235.
- Galli, E.; Vitel, E.; Schnell, F.; Le Rolle, V.; Hubert, A.; Lederlin, M.; Donal, E. Myocardial constructive work is impaired in hypertrophic cardiomyopathy and predicts left ventricular fibrosis. Echocardiography 2019, 36, 74–82.
- Chen, Y.-L.; Xu, T.-Y.; Chen, C.-H.; Xu, J.-Z.; Zhu, L.-M.; Li, Y.; Wang, J.-W. An Imaging Study on Cardiac Fibrosis in Primary Aldosteronism. Ruijin Hospital, Shanghai, China. 2021. manuscript in preparation.
- Young, W.F., Jr. Diagnosis and treatment of primary aldosteronism: Practical clinical perspectives. J. Intern. Med. 2019, 285, 126–148.
- Marzano, L.; Colussi, G.; Sechi, L.A.; Catena, C. Adrenalectomy Is Comparable with Medical Treatment for Reduction of Left Ventricular Mass in Primary Aldosteronism: Meta-Analysis of Long-Term Studies. Am. J. Hypertens. 2015, 28, 312–318.
- Ori, Y.; Chagnac, A.; Korzets, A.; Zingerman, B.; Herman-Edelstein, M.; Bergman, M.; Gafter, U.; Salman, H. Regression of left ventricular hypertrophy in patients with primary aldosteronism/low-renin hypertension on low-dose spironolactone. Nephrol. Dial. Transplant. 2013, 28, 1787–1793.
- Gaddam, K.; Corros, C.; Pimenta, E.; Ahmed, M.; Denney, T.; Aban, I.; Inusah, S.; Gupta, H.; Lloyd, S.G.; Oparil, S.; et al. Rapid Reversal of Left Ventricular Hypertrophy and Intracardiac Volume Overload in Patients with Resistant Hypertension and Hyperaldosteronism: A Prospective Clinical Study. Hypertension 2010, 55, 1137–1142.
- Rossi, G.P.; Cesari, M.; Cuspidi, C.; Maiolino, G.; Cicala, M.V.; Bisogni, V.; Mantero, F.; Pessina, A.C. Long-Term Control of Arterial Hypertension and Regression of Left Ventricular Hypertrophy With Treatment of Primary Aldosteronism. Hypertension 2013, 62, 62–69.
- Bernini, G.; Bacca, A.; Carli, V.; Carrara, D.; Materazzi, G.; Berti, P.; Miccoli, P.; Pisano, R.; Tantardini, V.; Bernini, M.; et al. Cardiovascular changes in patients with primary aldosteronism after surgical or medical treatment. J. Endocrinol. Investig. 2012, 35, 274–280.
- Giacchetti, G.; Ronconi, V.; Turchi, F.; Agostinelli, L.; Mantero, F.; Rilli, S.; Boscaro, M. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: An observational study. J. Hypertens. 2007, 25, 177–186.
- Indra, T.; Holaj, R.; Štrauch, B.; Rosa, J.; Petrák, O.; Šomlóová, Z.; Widimský, J. Long-term effects of adrenalectomy or spironolactone on blood pressure control and regression of left ventricle hypertrophy in patients with primary aldosteronism. J. Renin. Angiotensin. Aldosterone Syst. 2015, 16, 1109–1117.
- Chang, Y.; Liao, C.; Tsai, C.; Chen, C.; Pan, C.; Chen, Z.; Chen, Y.; Lin, L.; Chang, Y.; Wu, V.; et al. Left Ventricular Dysfunction in Patients With Primary Aldosteronism: A Propensity Score–Matching Follow-Up Study With Tissue Doppler Imaging. J. Am. Heart Assoc. 2019, 8, e013263.
- Chang, Y.-Y.; Tsai, C.-H.; Peng, S.-Y.; Chen, Z.-W.; Chang, C.-C.; Lee, B.-C.; Liao, C.-W.; Pan, C.-T.; Chen, Y.-L.; Lin, L.-C.; et al. KCNJ5 Somatic Mutations in Aldosterone-Producing Adenoma Are Associated With a Worse Baseline Status and Better Recovery of Left Ventricular Remodeling and Diastolic Function. Hypertension 2021, 77, 114–125.
- Dal Ferro, M.; De Paris, V.; Collia, D.; Stolfo, D.; Caiffa, T.; Barbati, G.; Korcova, R.; Pinamonti, B.; Zovatto, L.; Zecchin, M.; et al. Left Ventricular Response to Cardiac Resynchronization Therapy: Insights From Hemodynamic Forces Computed by Speckle Tracking. Front. Cardiovasc. Med. 2019, 6, 59.
- Sotomi, Y.; Okamura, A.; Iwakura, K.; Date, M.; Nagai, H.; Yamasaki, T.; Koyama, Y.; Inoue, K.; Sakata, Y.; Fujii, K. Impact of revascularization of coronary chronic total occlusion on left ventricular function and electrical stability: Analysis by speckle tracking echocardiography and signal-averaged electrocardiogram. Int. J. Cardiovasc. Imaging 2017, 33, 815–823.
- Tadic, M.; Cuspidi, C. Left ventricular strain and arterial hypertension: Is longitudinal strain ready for primetime? J. Clin. Hypertens. 2020, 22, 683–685.
- Ikonomidis, I.; Pavlidis, G.; Thymis, J.; Birba, D.; Kalogeris, A.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Endothelial Glycocalyx, Arterial Function, and Myocardial Work Index in Patients With Type 2 Diabetes Mellitus After 12-Month Treatment. J. Am. Heart Assoc. 2020, 9, e015716.
- Chen, Y.-L.; Xu, T.-Y.; Xu, J.-Z.; Zhu, L.-M.; Li, Y.; Wang, J.-G. A Prospective Comparative Study on Cardiac Alterations After Surgery and Drug Treatment of Primary Aldosteronism. Front. Endocrinol. 2021, 12, 770711.
