Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
The silica comes from the soil in the form of silicic acid, which the sugarcane plant absorbs and collects around the cellulose micro-compartments.
- sugarcane
- silica
- SiNPs
- silicon
1. Introduction
Valorization of sugarcane bagasse has been studied extensively over the years, with its niche applications in the sugar industry and bioenergy production. Silicon dioxide (SiO2), often known as silica, is a useful inorganic multifunctional chemical substance that is one of the basic materials. Silica can be found in nature as quartz, sand, or flint. Gel and amorphous forms are also possible, with both crystalline and amorphous forms found in the earth’s crust [1,2]. In their study, El Sayed and El-Sammi reported numerous silica content in different sources: Sorghum (88.75%), wheat (90.56%), corn (64.32%), bamboo (57.40%), bagasse (73%.00%), lantana (23%.38), sunflower (25.32%), rice husk (93.00%), rice straw (82.00%), and bread fruit tree (81.80%) [3].
The silica comes from the soil in the form of silicic acid, which the sugarcane plant absorbs and collects around the cellulose micro-compartments. The quantity of silicon in the soil influences silica concentration. Sugarcane roots play a key role in absorbing silicic acid from the soil and delivering it to the shoots, where it is stored as amorphous silica. Amorphous silica predominates in sugarcane bagasse ash with other metallic contaminants [2,3].
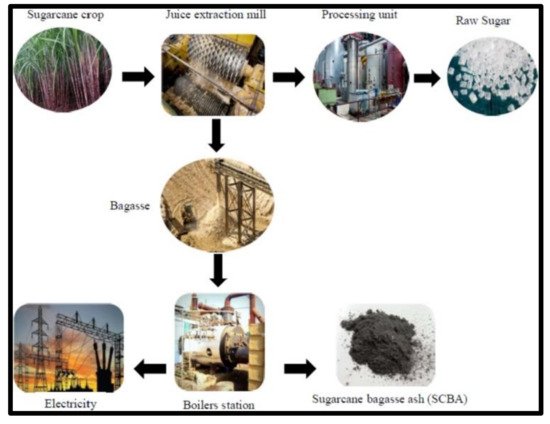
The sugarcane bagasse ash is rich with silica of about 73%, and it is economically feasible due to the conversion of raw material to the production of silica gels and powders [4,5]. The sugarcane bagasse has been investigated as the potential raw material and source of sugar, juice, and fuel in the ethanol industry (shown in Figure 1) and valuable material in the cementious industry [6].
Many industrial processes (shown in Figure 1) in the transformation of sugarcane into sugar and ethanol, namely, the burning process of the biomass for energy production, result in ashes with high impurity content, such as iron and aluminum oxides, hindering the process of obtaining silica with satisfactory purity values [6]. Thus, the resulting ashes require additional purification processes HCl acid leaching allied or not to a sol–gel method [6,7].
The silica nanocomposites (soluble silicates) are widely used in the glass, ceramic, and cement industries as a major component and in cosmetic, pharmaceutical, and detergent industries as bonding and adhesive agents. Notably, silica has been used as a major precursor in a variety of applications as inorganic and organometallic materials, which have desirable applications in synthetic chemistry as a catalyst, coating for electronic optical materials, and thin films [7].
Sugarcane Bagasse in South Africa
The sugarcane, shown in Figure 2, is an economical and strategic crop for the Mpumalanga and Kwazulu Natal, where the production is located, it is comprised of a substantial percentage of field crop gross farming income across the two provinces. To date, about 22,949 registered sugarcane growers annually produce an average of 20 million tons of sugarcane from 14 million supply areas, ballooning from southern Kwazulu-Natal to the Mpumalanga Lowveld [8].
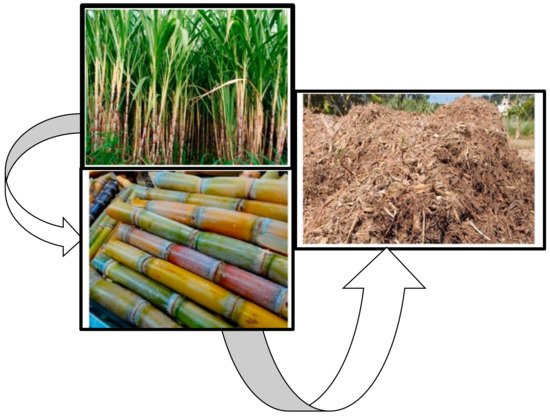
Figure 2. Images of a typical sugarcane plantation, stalks, and bagasse from the South African sugar industry [8].
Presently, the growers are represented by the Southern African Cane Growers Association (SACGA) and the South African Farmers Development Association (SAFDA). Thus, there are approximately 21,581 small-scale growers, of whom about 12,019 delivered cane in the 2018/2019 season, with the production at 9.33% of the total crop. This is composed of 116 consolidated units, including co-operatives, trusts, and projects, with the make-up of 7536 individual beneficiaries. There are 1368 large-scale growers, including 345 black emerging farmers, and they produce 81.17% of total sugarcane. Milling companies with their own sugar estates produce 9.17% of the crop [9].
Advanced studies have been ongoing to investigate the various ways for the production of silica from sugarcane bagasse using simple processing routes. The traditional routes moderately utilized for agricultural wastes to silica include synthetic routes, such as acid treatment, calcination, partial burning, leaching, enzymatic treatment, pyrolysis, hydrolysis, and sol–gel. Traditionally, silica is produced by reaction of sodium carbonate powder and quartz sand at higher temperatures to form sodium silicate, which will then react with sulfuric acid to precipitate silica [10].
This method is considered hazardous to the ecosystem since most of its by-products (sodium sulphate, carbon dioxide, and lots of wastewater) are detrimental to the environment.
2. Purification of Silicon
Production of silicon from several agricultural wastes is not usually obtained directly without passing through the refining process. Thus, purification of silicon is an essential part, especially in industrial solar-grade (SoG)-silicon. Due to the shortage of semiconductor-grade, which is expensive, it has been a great challenge for its production [49,50].
This later led to the new ways of upgrading the metallurgical-grade silicon at a lower cost though at the expense of loss of some of silicon. The upgraded metallurgical-grade silicon (UMG-Si) generally contains impurities, which affects the photovoltaic performance, namely, short-circuit current (JSC), open-circuit voltage (VOC), fill factor (FF), and power conversion efficiency in silicon-based solar cells [51,52,53,54].
Furthermore, the phosphorus diffusion gettering process has been widely utilized to improve the performance of Si solar cells. Moreover, the enhanced performance in the photovoltaic technology has been mainly due to improving the electrical properties of UMG-Si wafers and solar cells. The minority-carrier recombination lifetime and photovoltaic performance degradation of p-type silicon solar cells, as well as the effects of metallic contaminant type and concentration thereof (Al, Cu, Ni, and Fe), have been reported [55].
Therefore, the stable SoG-Si has led to the quest to find suitable purification methods and to reduce the production cost minimally without affecting the desired properties of the built solar cells. It is worth noting that these several purification methods are usually the simple preparation of SoG-Si from lower grades, mostly the MG-Si. Consequently, it has been shown that heat treatment could be used to essentially modify the morphology of purified silicon.
Presently, the solvent refining process has been proved to be an important purification method that is beneficiation to the cost due to its high purification efficiency and low processing temperature. It usually involves alloying silicon with another element to enable the solvation and solidification with recrystallization of silicon from the solvent at a much lower temperature than the melting point of silicon, subsequently followed by separation of the crystallized silicon [56,57]. Several other refining methods include directional solidification, fractional melting, leaching, slag treatment/gas blowing, electron beam melting, etc., [57].
This entry is adapted from the peer-reviewed paper 10.3390/app12052310
This entry is offline, you can click here to edit this entry!