Cytomegalovirus (CMV), a double-stranded DNA virus, is an important member of the Herpesviridae family. CMV infection can manifest as asymptomatic, constitutional symptoms or tissue-invasive diseases. The gastrointestinal (GI) tract is one of the most commonly involved systems and associated with 30% of tissue-invasive diseases among immunocompetent patients. CMV GI disease is defined on the basis of upper and/or lower GI symptoms, macroscopic mucosal lesions, and CMV documented in tissue by histopathology, virus isolation, rapid culture, immunohistochemistry (IHC), or DNA hybridization techniques. However, IHC staining has a higher sensitivity and specificity than routine HE staining.
- cytomegalovirus
- gastrointestinal tract
- prognostic factor
- antiviral therapy
1. Introduction
2. Clinical Presentation and Diagnostic Work-Up
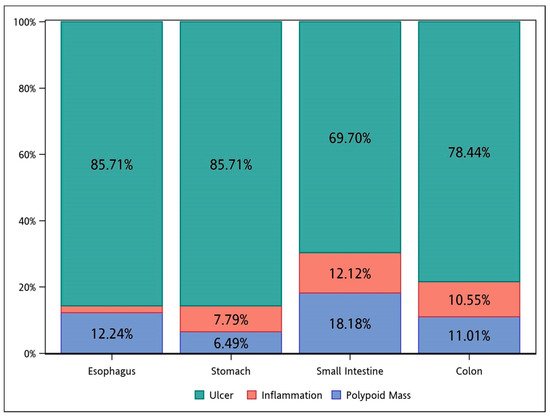
3. Treatment and Outcome
4. Cytomegalovirus Diseases of the Gastrointestinal Tract
This entry is adapted from the peer-reviewed paper 10.3390/v14020352
References
- Andrews, P.A.; Emery, V.; Newstead, C. Summary of the British Transplantation Society guidelines for the prevention and management of CMV disease after solid organ transplantation. Transplantation 2011, 92, 1181–1187.
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients—Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512.
- You, D.M.; Johnson, M.D. Cytomegalovirus infection and the gastrointestinal tract. Curr. Gastroenterol. Rep. 2012, 14, 334–342.
- Rafailidis, P.I.; Mourtzoukou, E.G.; Varbobitis, I.C.; Falagas, M.E. Severe cytomegalovirus infection in apparently immunocompetent patients: A systematic review. Virol. J. 2008, 5, 47.
- Azer, S.A.; Limaiem, F. Cytomegalovirus Colitis; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Gravito-Soares, E.; Almeida, N. Cytomegalovirus disease of the upper gastrointestinal tract: An emerging infection in immunocompetent hosts. GE-Port. J. Gastroenterol. 2017, 24, 259–261.
- O’Hara, K.M.; Pontrelli, G.; Kunstel, K.L. An introduction to gastrointestinal tract CMV disease. J. Am. Acad. Physician Assist. 2017, 30, 48–52.
- Wetwittayakhlang, P.; Rujeerapaiboon, N.; Wetwittayakhlung, P.; Sripongpun, P.; Pruphetkaew, N.; Jandee, S.; Chamroonkul, N.; Piratvisuth, T. Clinical features, endoscopic findings, and predictive factors for mortality in tissue-invasive gastrointestinal cytomegalovirus disease between immunocompetent and immunocompromised patients. Gastroenterol. Res. Pract. 2021, 2021, 8886525.
- Chaemsupaphan, T.; Limsrivilai, J.; Thongdee, C.; Sudcharoen, A.; Pongpaibul, A.; Pausawasdi, N.; Charatcharoenwitthaya, P. Patient characteristics, clinical manifestations, prognosis, and factors associated with gastrointestinal cytomegalovirus infection in immunocompetent patients. BMC Gastroenterol. 2020, 20, 22.
- Reddy, N.; Wilcox, C.M. Diagnosis & management of cytomegalovirus infections in the GI tract. Expert Rev. Gastroenterol. Hepatol. 2007, 1, 287–294.
- Yoon, J.; Lee, J.; Kim, D.S.; Lee, J.W.; Hong, S.W.; Hwang, H.W.; Hwang, S.W.; Park, S.H.; Yang, D.-H.; Ye, B.D.; et al. Endoscopic features and clinical outcomes of cytomegalovirus gastroenterocolitis in immunocompetent patients. Sci. Rep. 2021, 11, 6284.
- Bernard, S.; Germi, R.; Lupo, J.; Laverrière, M.H.; Masse, V.; Morand, P.; Gavazzi, G. Symptomatic cytomegalovirus gastrointestinal infection with positive quantitative real-time PCR findings in apparently immunocompetent patients: A case series. Clin. Microbiol. Infect. 2015, 21, 1121.e1–1121.e7.
- Le, P.-H.; Kuo, C.-J.; Wu, R.-C.; Hsu, J.-T.; Su, M.-Y.; Lin, C.-J.; Chiu, C.-T. Pancolitis associated with higher mortality risk of cytomegalovirus colitis in patients without inflammatory bowel disease. Ther. Clin. Risk Manag. 2018, 14, 1445–1451.
- Lee, J.S.; Yun, J.; Ham, S.; Park, H.; Lee, H.; Kim, J.; Byeon, J.-S.; Jung, H.-Y.; Kim, N.; Kim, D.H. Machine learning approach for differentiating cytomegalovirus esophagitis from herpes simplex virus esophagitis. Sci. Rep. 2021, 11, 3672.
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin. Infect. Dis. 2016, 64, 87–91.
- Gravito-Soares, E.; Almeida, N. Cytomegalovirus disease of the upper gastrointestinal tract: An emerging infection in immunocompetent hosts. GE-Port. J. Gastroenterol. 2017, 24, 259–261.
- Reddy, N.; Wilcox, C.M. Diagnosis & management of cytomegalovirus infections in the GI tract. Expert Rev. Gastroenterol. Hepatol. 2007, 1, 287–294.
