Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Surgery
Rex shunt, which was first put in use in 1992, has been considered as an ideal surgical method for the treatment of extra-hepatic portal venous obstruction (EHPVO) due to its reconstruction of the hepatopetal portal blood flow.
- extra-hepatic portal venous obstruction
- portal hypertension
- Rex shunt
- children
- treatment
1. Introduction
Extra-hepatic portal venous obstruction (EHPVO) is a condition that leads to prehepatic portal hypertension, where the forward hepatopetal flow of portal blood from the superior mesenteric vein, splenic vein, and coronary vein is impeded through a normal portal vein by a relative or complete obstruction [1]. Rex shunt was considered as an ideal surgical method for treating EHPVO due to its reconstruction of the portal blood flow into the liver [2]. Rex shunt refers to a surgery where the extra-hepatic portal blood is drained into the left branch of intra-hepatic portal vein in Rex recessus by bypassing or transposing a grafted vein, through which a hepatopetal portal blood is reconstructed. The word “Rex shunt” originated from the anatomic structure of Rex recessus (located between the liver segments III and IV and at the root of the hepatic round ligament) (Figure 1), in which the portal blood is re-shunted into the liver through the Rex shunt.
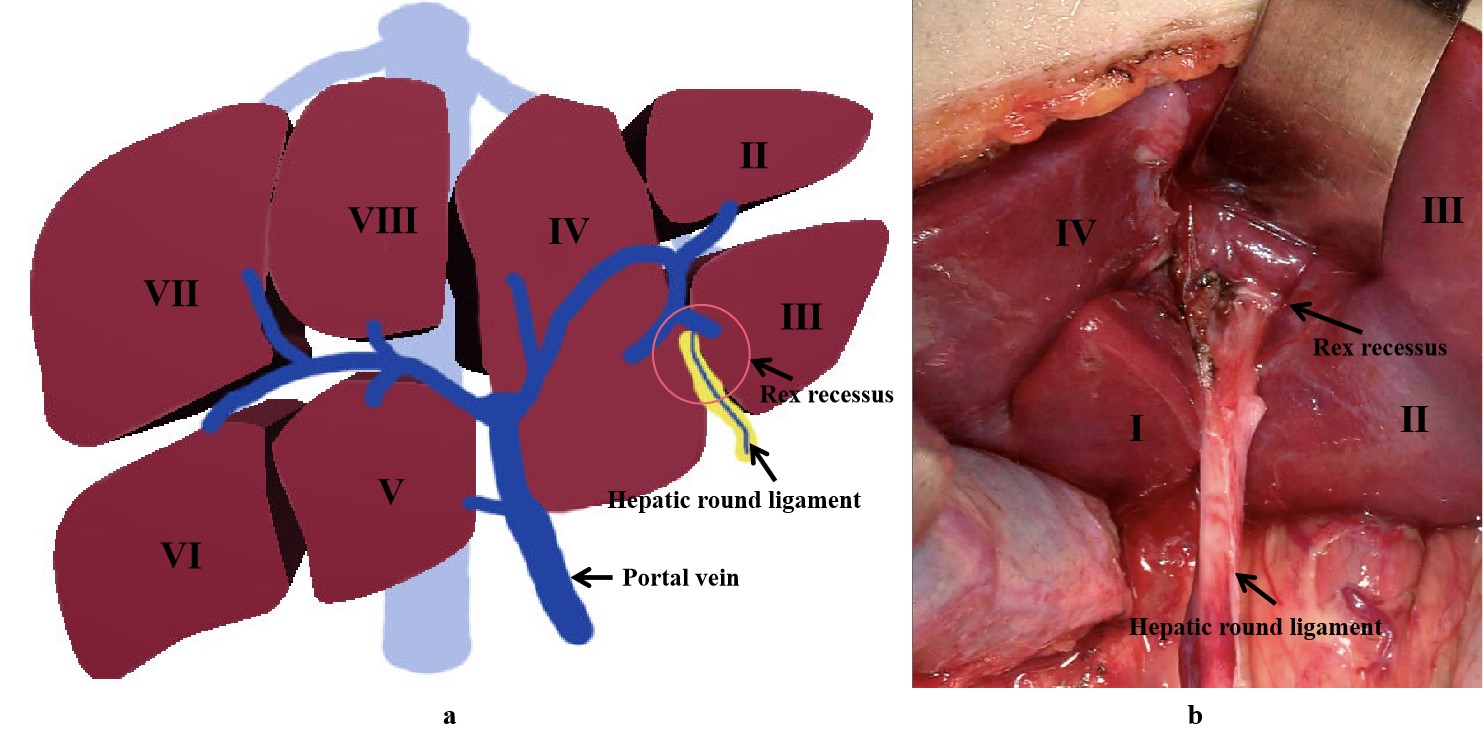
Figure 1. The location of Rex recessus ((a) a drawing picture; (b) an anatomic picture).
2. Surgical Method of Rex Shunt
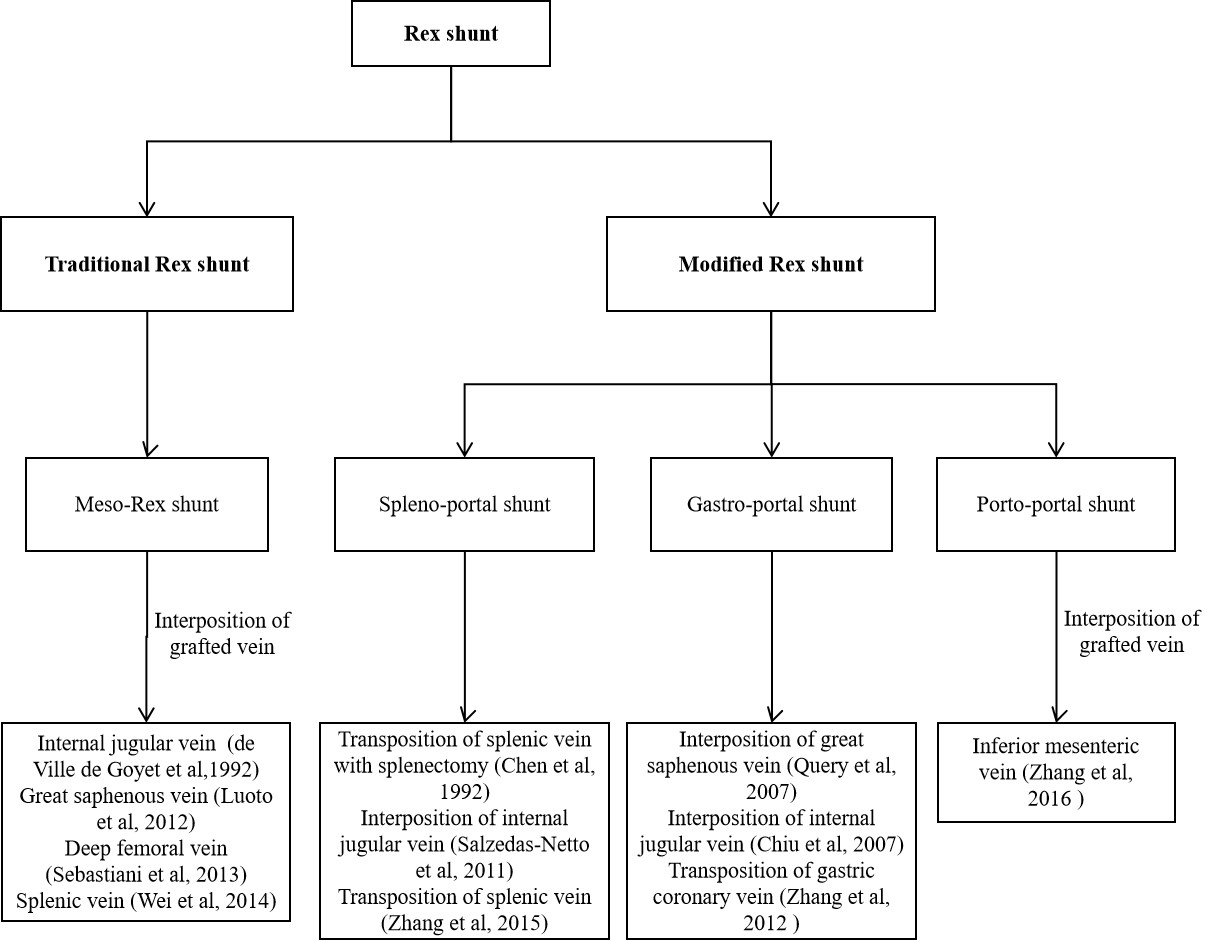
Surgical method of Rex shunt had experienced the modification of meso-Rex shunt, spleno-portal shunt, gastro-portal shunt, and porto-portal shunt since 1992 (Figure 2); however, its fundamental function is a reconstruction of the portal blood flow into the liver to restore the blood supply of the liver and relieve the extra-hepatic portal hypertension caused by EHPVO.
2.1. Meso-Rex Shunt
In 1992, de Ville et al. [3] first used a superior mesenteric vein to left portal vein shunt with the interposition of an internal jugular vein to treat EHPVO patients after liver transplantation (Figure 3a), which successfully alleviated the symptoms of extra-hepatic portal hypertension by reconstructing the hepatopetal portal blood flow [14]. Since then, this operation became known as “meso-Rex shunt” or “Rex shunt” [15], which are also used to distinguish the traditional Rex shunt from the modified Rex shunt using another bypass vein. In the meso-Rex shunt, a great saphenous vein, deep femoral vein, or splenic vein can also be used as a grafted vein [4,5,6].
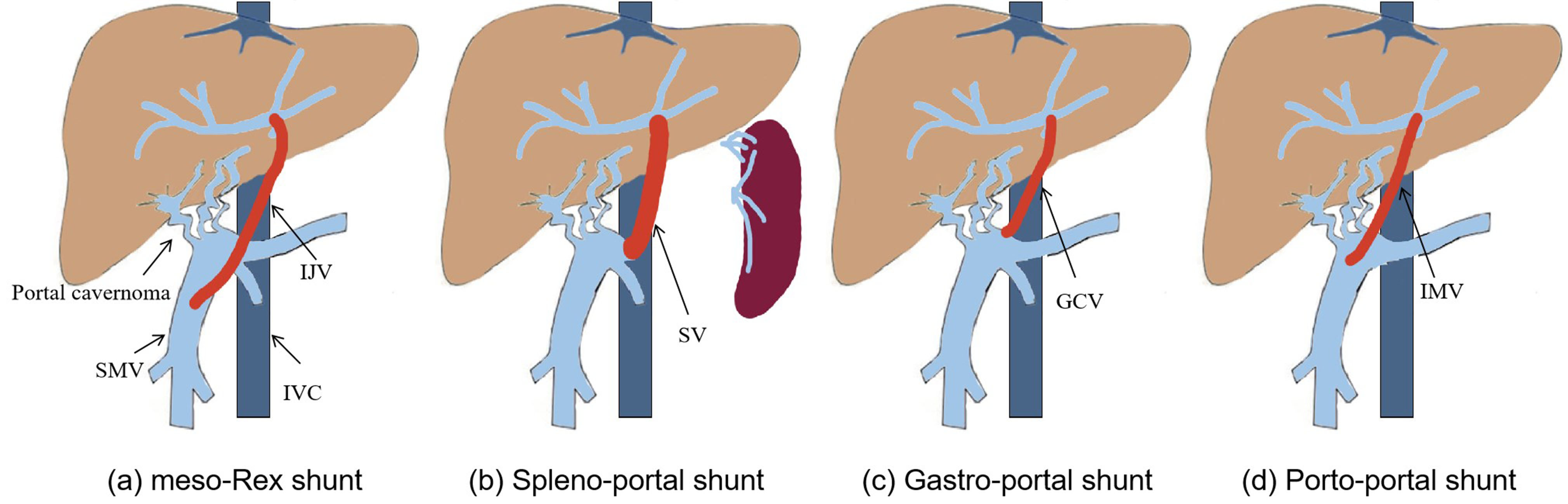
Figure 3. The Rex shunt including meso-Rex shunt (a), spleno-portal shunt (b), gastro-portal shunt (c) and porto-portal shunt (d) (IJV, internal jugular vein; SMV, superior mesenteric vein; IMV, inferior mesenteric vein; GCV, gastric coronary vein; SV, splenic vein; IVC, inferior vena cava).
2.2. Spleno-Portal Shunt
The first application time of the modified Rex shunt was very close to that of the traditional Rex shunt (meso-Rex shunt). In 1992, a splenic vein to left portal vein shunt (spleno-portal shunt) was first performed by Chen et al. [7] to treat a child with EHPVO after splenectomy, during which a splenic vein was directly transposed to the Rex recessus and anastomosed with the left portal vein. This Rex shunt avoided the transplantation of the internal jugular vein in meso-Rex shunt, but this operation was only used to treat EHPVO children undergoing splenectomy, which may be one of the causes for its limited application. In order to avoid splenectomy, a spleen preserving spleno-portal shunt was used in 2011 by Netto et al. [8] to treat a child with the portal vein and superior mesenteric vein thrombosis. An internal jugular vein was interposed between the splenic vein and the left portal vein during this procedure. This child was not suitable for meso-Rex shunt due to the superior mesenteric vein thrombosis. Whilst this surgical approach provided a new option for treating the children with superior mesenteric vein thrombosis, it inevitably required neck surgery. In order to avoid splenectomy and neck surgery, we used a spleen preserving spleno-portal shunt in 2015 to treat four children with EHPVO [9]. During this approach, the proximal end of the splenic vein was directly transposed to the Rex recessus and anastomosed with the left portal vein (Figure 3b). However, the spleno-portal shunt is less frequently used due to its difficulty of exposing and dissecting the splenic vein.
2.3. Gastro-Portal Shunt
A gastric coronary vein to left portal vein shunt (gastro-portal shunt) is another modified Rex shunt. In 2007, Query et al. [10] used a gastro-portal shunt with the interposition of a great saphenous vein to treat a child with EHPVO, during which a great saphenous vein was interposed between the gastric coronary vein and left portal vein. In 2007, Chiu et al. [11] interposed an internal jugular veinbetween the gastric coronary vein and left portal vein to treat two children with EHPVO. Nevertheless, transplantation of a great saphenous vein or an internal jugular vein would require leg or neck dissection, which increases the surgical incision except for the abdominal incision to perform Rex shunt. Consequently, we used a gastro-portal shunt without a grafted vein to treat eight EHPVO children in 2012 [12]. In this approach, the gastric coronary vein was dissected proximally to the esophageal hiatus, and its distal end was transposed to the Rex recessus and anastomosed with the left portal vein (Figure 3c). This operation is widely used in treating EHPVO due to its reduction of vascular anastomotic number and avoidance of grafted veins in our center [16]. However, the gastro-portal shunt is not suitable for most EHPVO due to the requirement of the gastric coronary vein (no less than 5 mm in diameter and a suitable length) [16].
2.4. Porto-Portal Shunt
The inferior mesenteric vein is an important branch of the portal venous system that collects the blood of the left half of the colon, sigmoid colon, and rectum. Because there are many communicating venous arches, transection of the inferior mesenteric vein does not affect the blood supply of the colon and rectum. Based on this anatomy, the inferior mesenteric vein is a suitable graft. In 2003, Ates et al. [17] performed an inferior mesenteric vein to left portal vein shunt in order to treat a child with EHPVO, where the proximal end of the inferior mesenteric vein was transposed to the Rex recessus and anastomosed with the left portal vein, avoiding transplantation of other blood vessels and simplifying the operation. Nevertheless, the inferior mesenteric vein needs to cross the front of the pancreas and pass through the back of the stomach in its transposition to the Rex recessus, which may affect the patency of the bypass vein due to angulation and compression from surrounding tissue. In addition, the inferior mesenteric vein requires a sufficient length. In 2016, we used a portal cavernoma to left portal vein shunt with the interposition of the inferior mesenteric vein (porto-portal shunt) for the treatment of nine children with EHPVO [13], during which a grafted inferior mesenteric vein was interposed between the dilated part of portal cavernoma and the left portal vein (Figure 3d), thus reducing the length of grafted vein compared with the meso-Rex shunt and avoiding other surgical incisions. The porto-portal shunt is suitable for almost all EHPVO due to natural conditions of inferior mesenteric vein as a grafted vein, which was considered an optimal modified Rex shunt in our center [16].
3. The Indications of Rex Shunt
3.1. Surgical Indications
Due to its function of reconstructing portal blood flow into the liver, Rex shunt is often used to treat EHPVO caused by thrombosis and tumor embolism and can also be used for portal vein reconstruction during liver transplantation. In 2006, Superina et al. [2] formulated a surgical guideline of EHPVO, which identified the preconditions for Rex shunt, including the diagnosis of EHPVO by portal venography, patent left portal vein by intra-hepatic portal venography or surgical exploration and without liver diseases (liver fibrosis or cirrhosis). According to the surgical guidelines of EHPVO [2], Rex shunt should be timely performed when there is an absolute indication including medically/endoscopically refractory variceal hemorrhage, severe hypersplenism (platelet count < 10,000), recurrent complications including non-variceal hemorrhage or infections, symptomatic and medically refractory porto-systemic encephalopathy, hepato-pulmonary syndrome and porto-pulmonary hypertension. Rex shunt can be postponed when there is a relative indication including symptomatic splenomegaly, unacceptable activity restrictions because of splenomegaly, large varices and poor access to health care, neuro-cognitive testing suggestive of porto-systemic encephalopathy, portal biliopathy and unexplained failure to thrive or delay in sexual development. Performing a prophylactic Rex shunt is also a feasible option. A negative correlation was found between shunt blood flow and operative age [18], which suggested that delaying operative age did not result in a better prognosis. Therefore, when EHPVO is diagnosed, and the indications for Rex shunt are met, Rex shunt should be timely performed.
3.2. Pre- and Intra-Operative Assessment
The diagnosis of EHPVO should be identified before making the decision of performing Rex shunt. Commonly, the abdominal ultrasound (US) and enhanced computed tomography (CT) or magnetic resonance angiography (MRA) are used to evaluate the anatomy of portal venous system. Although US is a simple and non-invasive diagnostic method, the accuracy of US in diagnosing EHPVO decreases due to the interference of abdominal gas or the existence of a dilated collateral straight portal vein. Abdominal CT or MRA can improve the accuracy of EHPVO diagnosis [19]. Furthermore, a retrograde portal venography is a necessary radiological detection due to its gold standard for diagnosing EHPVO. Except for diagnosis of EHPVO, the purpose of pre-operative assessment is to visualize the intrahepatic portal and hepatic veins, superior mesenteric vein, splenic vein, renal veins, and inferior vena cava above and below the liver. Finally, hemotological tests, including liver function tests, routine blood test, and coagulation function, should be performed to exclude liver disease and identify hypercoagulable states [2].
An intra-operative portal venography should be performed for children without a retrograde portal venography before surgery [9,12,16]. During the operation, the intra- and extra-hepatic portal vein system are visualized to identify the diagnosis of EHPVO and the patency of left portal vein, which provides a reliable imaging basis for selecting a suitable bypass vein and avoiding an unnecessary operation [20]. Therefore, portal venography has an important role in the performance of Rex shunt. In addition, liver biopsy should be performed to exclude significant intrinsic liver disease. The following conditions need to be satisfied for the performance of a modified Rex shunt: the diameter of the grafted vein is no less than 5 mm, and the length of the grafted vein is suitable for a tension-free anastomosis [9,12,16].
The interdisciplinary care is also very important, as gastroenterologists, surgeons, and pediatricians should be involved in diagnosis and management of EHPVO.
4. The Strengths of Rex Shunt
Therapeutic options for EHPVO include surgical methods and radiological interventions. The surgical methods include devascularization, endoscopic esophagogastric vein ligation and sclerotherapy, portosystemic shunt, and Rex shunt.
4.1. Devascularization, Endoscopic Esophagogastric Vein Ligation, and Sclerotherapy
The devascularization and endoscopic esophagogastric vein ligation and sclerotherapy can immediately stop upper gastrointestinal bleeding. Yet, the recurrence of esophagogastric varices is inevitable due to portal hypertension, which results in a high recurrence rate. Therefore, the devascularization and endoscopic esophagogastric vein ligation and sclerotherapy are usually used as an emergency method for temporary hemostasis [21,22]. Although splenectomy can effectively relieve hypersplenism, it cannot improve portal hypertension. In addition, the incidence of infection and portal vein thrombosis after splenectomy in children significantly increases [23,24,25,26]. Therefore, this approach is not recommended for treating portal hypertension in children.
4.2. Portosystemic Shunt
Portocaval shunt, proximal splenorenal shunt, and mesocaval shunt as non-selective shunt have been widely used to treat portal hypertension [22], which create a direct communication between the portal system and the systemic circulation, and depending on the flow, a full diversion can be achieved, with a consequent fall in the portal pressure. The duration from undergoing a surgery to the average life span in children is significantly longer than that of adults. Therefore, except for the surgical effectiveness, the postoperative quality of life should be considered after surgery for children. Although the non-selective shunt can significantly reduce portal pressure, it is prone to hepatic encephalopathy and liver failure due to the reduction of portal blood flow into the liver, which will worsen the postoperative quality of life. Therefore, the non-selective shunt is not an ideal surgery for EHPVO in children.
Distal splenorenal shunt (DSRS or Warren operation) as a selective shunt, which preserves portal and mesenteric blood flow to the liver, have been used with success to treat bleeding varices and hypersplenism and has been shown to reduce postoperative encephalopathy with equivalent long-term mortality and rebleeding rates. However, it cannot reconstruct the portal blood flow into the liver, and liver injury is inevitable due to the decreased portal blood flow into the liver. Therefore, the DSRS should be done at the time of surgery if the meso-Rex shunt cannot be completed because of anatomical issues [2].
4.3. Radiological Interventions
Transjugular intrahepatic portosystemic stent-shunt (TIPSS), as an important interventional therapy, is effective for treating hepatic or post-hepatic portal hypertension due to its advantages of avoiding a major abdominal surgery intervention and preserving the abdominal intact for possible transplantation. However, experience of TIPSS in children remains limited to case reports because it is more difficult in small patients [27]. Furthermore, portal cavernoma remains a relative contra-indication for TIPSS, and the presence of cavernoma has been associated with a significantly high failure rate of TIPSS [28]. Therefore, children with portal cavernoma are considered for TIPSS only if the meso-Rex shunt is not technically feasible [29].
A percutaneous transhepatic angioplasty/stenting is another interventional treatment for EHPVO (thrombosed portal vein), during which the portal trunk is recanalized through the placement of stent or balloon dilation in the portal vein. However, complete stent recanalization of EHPVO was possible only in one out of five children with the placement of stent due to difficulty attributable to the small caliber of veins [30]. In addition, EHPVO differs from common idiopathic prehepatic portal hypertension in that the intrahepatic portal system is large enough in diameter to allow transhepatic access and intravascular maneuvers. Therefore, though intervention is feasible for the treatment of EHPVO, it is rarely used in the treatment of children with EHPVO.
4.4. Rex Shunt
In addition to reducing portal pressure, Rex shunt avoids the occurrence of liver dysfunction caused by the insufficient blood supply to the liver by reconstructing the blood flow into the liver. Compared with portosystemic shunt, Rex shunt could significantly improve upper gastrointestinal bleeding, thrombocytopenia, coagulation function, serum albumin level, and body weight [31], thus indicating that Rex shunt can improve liver metabolic function. The deficiency of anticoagulant factors (protein C, protein S, and antithrombin III) was closely related to the occurrence of deep venous thrombosis (including portal vein thrombosis). Rex shunt could alleviate the deficiency of protein C, protein S, and antithrombin III in EHPVO by restoring the hepatopetal blood flow to improve the coagulation function and prevent bypass vein thrombosis [32].
The quality of life in children with EHPVO may be affected by splenomegaly-related pain, growth retardation, and portal cavernoma cholangiopathy (PCC). In one previous study, 54% of EHPVO children suffered from growth retardation due to malabsorption, deprivation of hepatotropic factors, chronic anemia, and growth hormone resistance [33]. Yet, Rex shunt can improve the growth parameters due to restoring blood supply to the liver [34]. According to a previous study, 92% (66/72) children with EHPVO had PCC due to compression on the biliary tree from long-standing portal cavernoma in the biliary and peribiliary region, where 85% of children were asymptomatic, and 7% were symptomatic [35]. The liver biochemistry was completely normalized after Rex shunt in 2/8 children with symptomatic PCC due to its decompression of portal cavernoma through restoring the blood flow into the liver [36]. Therefore, Rex shunt can improve the quality of life in children with EHPVO.
Progressive deterioration of liver functions and ascites may develop with increasing age, prolonged disease duration, and portal biliopathy in EHPVO due to decreasing hepatopetal portal blood flow [37]. On the other hand, Rex shunt can improve liver dysfunction. Therefore, Rex shunt should be performed as soon as possible, as postponing the performance of Rex shunt is not a wise choice [1].
In addition, no differences were reported in shunt complications, mortality, or gastrointestinal bleeding after surgery between meso-Rex shunt and portosystemic shunt in a meta-analysis, which indicated that meso-Rex shunt did not increase shunt complications, mortality, or gastrointestinal bleeding after surgery [38].
This entry is adapted from the peer-reviewed paper 10.3390/children9020297
This entry is offline, you can click here to edit this entry!