Among Eulipotyphla, the Erinaceinae subfamily containing the well-known hedgehogs of Eurasia and Africa deserves special attention. The helminth fauna of the Northern white-breasted hedgehog Erinaceus roumanicus was studied in the Republic of Mordovia (Russia) for the first time. A total of 54 parasite species were recorded across Erinaceus europaeus, E. roumanicus, E. concolor and E. amurensis. Among all the studied species of hedgehogs, E. europaeus (35 species) and E. roumanicus (36) have the richest helminth faunas. The diversity of the parasite communities of Erinaceus spp. is due to the wide distribution and varied diet of these mammals. Most of the helminths found in hedgehogs are transmitted along trophic chains.
- Erinaceus spp.
- parasitic worms
- Western European hedgehog
- Northern white-breasted hedgehog
- Southern white-breasted hedgehog
- Amur hedgehog
- Palaearctic
1. Introduction
2. Helminths of Erinaceus roumanicus in Mordovia (Russia)
| Helminth Species | Location in Host | P, % | IR, Spec. | MA |
|---|---|---|---|---|
| Trematoda Isthmiophora melis (Schrank, 1788) |
small intestine | 13.0 | 2–86 | 4.0 |
| Strigea strigis (Schrank, 1788), metacercaria | mesentery around oesophagus and trachea | 4.3 | 2 | 0.1 |
| Cestoda Hymenolepis erinacei (Gmelin, 1789) |
small intestine | 52.2 | 1–97 | 7.8 |
| Nematoda Aonchotheca erinacei (Rudolphi, 1819) |
stomach, small intestine | 56.5 | 1–149 | 19.5 |
| Physaloptera clausa Rudolphi, 1819 | stomach | 100 | 9–420 | 77.4 |
| Crenosoma striatum Zeder, 1800 | bronchi | 8.7 | 3–18 | 0.9 |
| Physocephalus sexalatus (Molin, 1860), juv. | walls of stomach and small intestine | 13.0 | 8–177 | 8.6 |
| Agamospirura minuta Sharpilo, 1963 | gastric mucosa and first third of small intestine | 4.3 | 3 | 0.1 |
AcanthocephalaNephridiorhynchus major (Bremser, 1811) |
small intestine | 4.3 | 2 | 0.1 |
3. Comparative Analysis of the Helminth Fauna of Erinaceus spp.
The helminth fauna of Erinaceus spp. in Palaearctic includes 54 species: 14 trematodes, 8 cestodes, 27 nematodes and 7 acanthocephalans. Among all the studied species of hedgehogs, E. europaeus (35 species) and E. roumanicus (36) have the richest helminth faunas. A significantly smaller number of parasite species was found in E. concolor (12) and E. amurensis (4). The greatest richness of the helminth fauna in hedgehogs was in Russia and Belarus, where 17 species of parasites were found in each country (Figure 1). Thirteen species of parasites were found in hedgehogs both in Italy and in Ukraine. Twelve species of parasitic worms were recorded in hedgehogs from the Czech Republic. Eleven helminth species were reported in hedgehogs in the UK, Germany, and Bulgaria, respectively; and, there were a reported 10 speciesin Spain and 9 speciesin Switzerland (Figure 1).
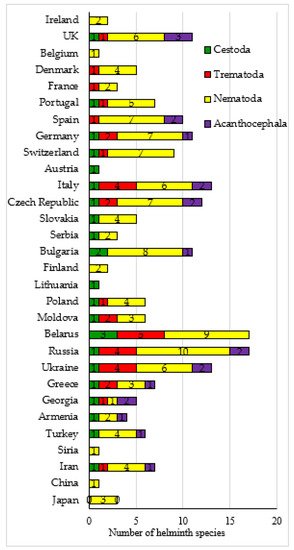
Comparative analysis of the helminth species composition in Erinaceus spp. from different countries showed, on the one hand, the originality of the parasite faunas of each hedgehog species, on the other, the similarity of the helminth communities of Erinaceus spp. from different regions of the Palaearctic. The originality of the helminth fauna of hedgehogs is achieved by parasitizing them with accidental or non-specific helminths or locally distributed helminths. Thus, in E. europaeus and E. roumanicus, 15 species of parasites were noted each, which are not found in other species of hedgehogs. The trematode B. recurva was found only in E. concolor, and the nematode Monovaria sp. in E. amurensis (Table 1). As a result, the average and low degree of similarity of the helminth faunas of different species of Erinaceus hedgehogs index was noted according to the Jaccard index.
The similarity of the helminth fauna of hedgehogs in certain studied regions is defined as a wide distribution of specific helminth species of Erinaceus spp. (C. striatum, H. erinacei, A. erinacei, P. clausa, and others), and the geographical proximity of study areas. Thus, the highest similarity was noted in the helminth composition of different hedgehog species from the same territory: E. europaeus and E. roumanicus from the Czech Republic, Poland, and Slovakia. As well as hedgehogs from Serbia and Slovakia (0.75–0.86), Germany and Switzerland (0.70), Czech Republic and Germany (0.70) (Figure 2).
A high similarity in the helminth fauna of hedgehogs from countries far from each other was noted only when comparing the parasites of one species of hedgehogs (in E. europaeus from Finland and Ireland – 0.67). As a rule, the helminth faunas of different species of hedgehogs from distant countries has a low similarity (E. europaeus from Spain and E. roumanicus from Russia – 0.50, E. europaeus from Russia and E. roumanicus from Bulgaria – 0.30)4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/d14030165
References
- Otso Huitu; Kai Norrdahl; Erkki Korpimäki; Competition, predation and interspecific synchrony in cyclic small mammal communities. Ecography 2004, 27, 197-206, 10.1111/j.0906-7590.2003.03684.x.
- Rutovskaya, M.V.; Aleksandrov, A.N.; Podshivalina, V.N.; Soboleva, A.S.; Glushenkov, O.V.; Habitat conditions of Desmana moschata (Talpidae, Eulipotyphla, Mammalia) in the buffer zone of the Prisurskiy State Nature Reserve (Russia). Nature Conservation Research 2020, 5, 36-46, 10.24189/ncr.2020.011.
- Bashinskiy, I.V.; Beaver impact on water coverage of forest-steppe territories (Penza Region, European Russia). Nature Conservation Research 2021, 6, 88-97, 10.24189/ncr.2021.016.
- Lazutkin, A.N.; Long-term monitoring of red-backed voles number in Magadan Nature Reserve in 1980–2021. Proceedings of Mordovia State Nature Reserve 2021, 29, 319-325, .
- Yakimova, A.E.; Irina S. Gaidysh; The species composition and abundance of terrestrial small mammals in the Finnish-Russian Friendship Nature Reserve. Nature Conservation Research 2021, 6, 127-136, 10.24189/ncr.2021.028.
- V. A. Gremyachikh; D. A. Kvasov; E. S. Ivanova; Patterns of mercury accumulation in the organs of bank vole Myodes glareolus (Rodentia, Cricetidae). Biosystems Diversity 2019, 27, 329-333, 10.15421/011943.
- Lebedinskii, A.A.; Noskova, O.S.; Dmitriev, A.I.; Post-fire recovery of terrestrial vertebrates in the Kerzhensky State Nature Biosphere Reserve (Central Volga Region, Russia). Nat. Conserv. Res 2019, 4, 45-56, 10.24189/ncr.2019.049.
- Vekhnik, V.A.; Comparative analysis of biology and ecology of Glis glis (Gliridae, Rodentia) in the Zhiguli State Nature Reserve (Russia) and adjacent territories. Nature Conservation Research 2020, 5, 1-20, 10.24189/ncr.2020.001.
- Robert Poulin; Serge Morand; The Diversity of Parasites. The Quarterly Review of Biology 2000, 75, 277-293, 10.1086/393500.
- Alessandro Balestrieri; Andrea Gazzola; Giulio Formenton; Luca Canova; Long-term impact of agricultural practices on the diversity of small mammal communities: a case study based on owl pellets. Environmental Monitoring and Assessment 2019, 191, 725, 10.1007/s10661-019-7910-5.
- Laurent Ahissa; Bertin K. Akpatou; Hilaire K. Bohoussou; Blaise Kadjo; Inza Koné; University of Man; Swiss Centre for Scientific Research in Côte d’Ivoire; Species composition and community structure of terrestrial small mammals in Tanoé-Ehy Swamp Forest (South-East Ivory Coast): implication for conservation. Nature Conservation Research 2020, 5, 53-63, 10.24189/ncr.2020.005.
- Margarita I. Kononova; Yuri A. Prisniy; Helminthes of mouse-like rodents in the Belogorye State Nature Reserve (Russia). Nature Conservation Research 2020, 5, 11-18, 10.24189/ncr.2020.036.
- Nadezhda Kirillova; Alexander Ruchin; Alexander Kirillov; Helminths in Myomorph Rodents (Rodentia, Myomorpha) from the National Park “Smolny” and Its Surroundings (European Russia). Forests 2021, 12, 1510, 10.3390/f12111510.
- Boris V. Romashov; Irina M. Odoevskaya; Natalya B. Romashova; Nona A. Golubova; Ecology of trichinellosis transmission in the Voronezh State Nature Reserve and adjacent areas, Russia. Nature Conservation Research 2021, 6, 1-15, 10.24189/ncr.2021.023.
- Reeve, N.J. . Hedgehogs; T & AD Poyser Natural History: London, UK, 1994; pp. 313.
- Anni Rautio; Marja Isomursu; Anu Valtonen; Varpu Hirvelä-Koski; Mervi Kunnasranta; Mortality, diseases and diet of European hedgehogs (Erinaceus europaeus) in an urban environment in Finland. Mammal Research 2015, 61, 161-169, 10.1007/s13364-015-0256-7.
- Jourde, P. . Le hérisson d’Europe; Delachaux et Niestlé: Paris, France, 2013; pp. 207.
- Hutterer, R. Order Erinaceomorpha; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University Press: Baltimore, USA, 2005; pp. 220-311.
- Aulagnier, S.; Haffner, P.; Mitchell-Jones, A.J.; Moutou, F.; Zima, J.. Mammals of Europe, North Africa and the Middle East; A and C Black Publishers: London, UK, 2009; pp. 272.
- Holz, H.; Studies on European hedgehogs. Z. Zool. Syst. Evolut.-forsch. 1978, 16, 148-165, .
- Krystufek, B.; The distribution of hedgehogs (Erinaceus L., 1758, Insectivora, Mammalia) in Western Yugoslavia. Biosistematika 1983, 9, 71-78, .
- J. M. Seddon; F. Santucci; N. Reeve; G. M. Hewitt; Caucasus Mountains divide postulated postglacial colonization routes in the white-breasted hedgehog, Erinaceus concolor. Journal of Evolutionary Biology 2002, 15, 463-467, 10.1046/j.1420-9101.2002.00408.x.
- R. S. Sommer; When east met west: the sub‐fossil footprints of the west European hedgehog and the northern white‐breasted hedgehog during the Late Quaternary in Europe. Journal of Zoology 2007, 273, 82-89, 10.1111/j.1469-7998.2007.00302.x.
- Amori, G. Erinaceus europaeus. The IUCN Red List of Threatened Species 2016: e.T29650A2791303. 2016. Available at https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T29650A2791303.en. (accessed 13 February 2020)
- A. S. Bogdanov; A. A. Bannikova; Yu. M. Pirusskii; N. A. Formozov; The first genetic evidence of hybridization between West European and Northern white-breasted hedgehogs (Erinaceus europaeus and E. roumanicus) in Moscow region. Biology Bulletin 2009, 36, 647-651, 10.1134/s106235900906017x.
- Cassola, F. Erinaceus amurensis. The IUCN Red List of Threatened Species 2016: e.T40604A22325640. 2016. Available at http://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T40604A22325640.en (accessed 20 January 2022)
- Riley, P.Y.; Chomel, B.B.; Hedgehog zoonoses. Emerg. Infect. Dis. 2005, 11, 1-5, .
- Keymer, I.F.; Gibson, E.A.; Reynolds, D.J.; Zoonoses and other findings in hedgehogs (Erinaceus europaeus): a survey of mortality and review of the literature. Vet. Rec. 1991, 128, 245-252, .
- Egli, E. Comparison of physical condition and parasite burdens in rural, suburban and urban hedgehogs Erinaceus europaeus: Implications for conservation. M.Sc. Thesis, Bern University, Switzerland, 2004
- Andreyko, O.F. . . Parasites of mammals of Moldova; Stiintsa: Kishinev, Moldova, 1973; pp. 183.
- Fameree, L.; Cotteleer, C.; Van den Abbeele, O.; Epidemological and sanitary importance of Trichinosis in wild animals in Belgium – a summary of investigations 1979–1981. Schweiz. Arch. Tierheilkd. 1982, 124, 401-412, .
- Genov, T. . Helminths of insectivores and rodents in Bulgaria; Bulgarian Academy of Sciences : Sofia, Bulgaria, 1984; pp. 348.
- Bychkova, E.I.; Akimova, L.N.; Degtyarik, S.M., Yakovich, M.M.. Helminths of vertebrares and man in Belarus; Belarusskaya Nauka : Minsk, Belarus, 2017; pp. 316.
- Gabriella Gaglio; Simon Allen; Lee Bowden; Mark Bryant; Eric Morgan; Parasites of European hedgehogs (Erinaceus europaeus) in Britain: epidemiological study and coprological test evaluation. European Journal of Wildlife Research 2010, 56, 839-844, 10.1007/s10344-010-0381-1.
- Wroot, A.J.; Foraging in the European hedgehog, Erinaceus europaeus. Mammal. Rev. 1985, 15, 2, .
- A. Faltýnková; V. Našincová; L. Kablásková; Larval trematodes (Digenea) of the great pond snail, Lymnaea stagnalis (L.), (Gastropoda, Pulmonata) in Central Europe: a survey of species and key to their identification.. Parasite 2007, 14, 39-51, 10.1051/parasite/2007141039.
