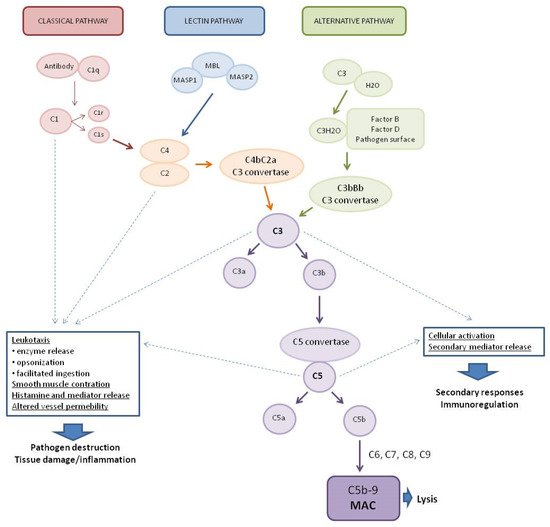
The complement system (CS) is part of the human immune system, consisting of more than 30 proteins that play a vital role in the protection against various pathogens and diseases, including viral diseases. Activated via three pathways, the classical pathway (CP), the lectin pathway (LP), and the alternative pathway (AP), the complement system leads to the formation of a membrane attack complex (MAC) that disrupts the membrane of target cells, leading to cell lysis and death. Due to the increasing number of reports on its role in viral diseases, which may have implications for research on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
- complement system
- viral diseases
- respiratory diseases
1. The Complement System (CS)
| Factor | Function | References | |
|---|---|---|---|
| C1 Esterase Inhibitor (C1-INH) | Plasma serine proteinase inhibitor (serpin). Binds to activated C1r and C1s, irreversibly inhibiting their activity, inhibition of classical pathway. Inhibits MASP-1 and MASP-2 | [26][27] | |
| Factor I (FI) | Protease inactivating C4b and C3b with cofactors | [28] | |
| Soluble Regulatory Proteins: C4b-Binding Protein and factor H (FH) | Cofactors for factor I. Accelerates the decomposition of the C4b2a and C3bBb complex. It is necessary for the regulation of C3 activity | [27][29][30][31] | |
| Membrane Regulatory Proteins | Protect cells from complement mediated lysis | [28][32] | |
| Decay-accelerating Factor (DAF) (CD55) | Factor accelerating the decomposition of C3 and C5 convertases | ||
| Membrane cofactor protein (MCP, CD46) |
Binds components C3b and C4b in the free state or in convertase. | ||
| Properdin | Stabilizes C3 and C5 convertases | [33][34] | |
| Soluble MAC Inhibitors | [27][32] | ||
| Vitronectin | Binds MAC and prevents the complex from being inserted into the cell membrane | ||
| Clusterin | Inactivates MAC with vitronectin | ||
| Membrane MAC Inhibitor CD59 | The primary membrane-bound inhibitor of the MAC. It binds to C8 and C9, preventing the incorporation and polymerization of C9 | [27][32] | |
2. The Complement System at the Crossroads of the Innate and Adaptive Immune Response
3. Antiviral Activity of the Complement System and Viral Strategies for Reducing the Complement System Action
| Disease | Virus | Family | Strategies Evasion | References |
|---|---|---|---|---|
| Influenza | Influenza viruses |
Orthomyxoviridae | Virus acquires CD59 on the surface and inhibits C1q-mediated recognition of virions Inhibition of neutralization by blocking the interaction of C1q with antibodies bound to the viral surface Inability of human C3b to recognize the surface of the virus and its opsonization |
[60][61][62] |
| Severe Acute Respiratory Syndrome (SARS) Middle East Respiratory Syndrome (MERS) Coronavirus disease (COVID-19) |
SARS-CoV MERS-CoV SARS-CoV-2 |
Coronaviridae | No data | - |
| Viral Lower Respiratory Tract Illiness |
Respiratory syncytial virus (RSV) |
Pneumoviridae | Transcriptional regulation of complement proteins |
[63] |
| Hepatitis B (HB) | Hepatitis B virus (HBV) |
Hepadnaviridae | The HBV X protein (HBx) upregulates CD59 and C4b-binding protein α (C4BPα), which inhibit the formation of MAC and provides protection from complement-mediated cytolysis | [64][65] |
| Ebola Virus Disease (EVD) | Ebola virus (EBOV) | Filoviridae | No data | - |
| Flaviviridae | Non-structural protein NS1 function as a regulator of the complement system. NS1 directly binds C4b binding protein (C4BP) on the surface of infected cells resulting in inhibition of complement activation in all pathways and MAC formation | [66] | ||
| Dengue | Dengue virus (DENV 1-4) | NS1 competitively binds to MBL, which prevents the later from recognizing and neutralizing the virus. NS1 binds clusterin/vitronectin on the surface of infected cells, resulting in the inhibition of complement activation in all pathways and MAC formation |
[67][68][69] | |
| Zika Virus Disease (ZVD) | Zika virus (ZIKV) | Incorporation into the viral envelope the of regulatory protein CD55 which contributes to virus stability and helps to avoid complement-dependent virolysis | [70] | |
| West Nile Fever (WNF) | West Nile virus (WNV) |
NS1 directly binds and recruits FH to the surface of infected cells resulting in the inhibition of complement activation in all pathways and MAC formation | [71] |
This entry is adapted from the peer-reviewed paper 10.3390/biom12020226
References
- Mathern, D.R.; Heeger, P.S. Molecules Great and Small: The Complement System. Clin. J. Am. Soc. Nephrol. 2015, 10, 1636–1650.
- Walport, M.J. Complement. First of two parts. N. Engl. J. Med. 2001, 344, 1058–1066.
- Cochrane, C.G.; Unanue, E.R.; Dixon, F.J. A Role of Polymorphonuclear Leukocytes and Complement in Nephrotoxic Nephritis. J. Exp. Med. 1965, 122, 99–116.
- Mastellos, D.; Morikis, D.; Isaacs, S.N.; Holland, M.C.; Strey, C.W.; Lambris, J.D. Complement: Structure, functions, evolution, and viral molecular mimicry. Immunol. Res. 2003, 27, 367–386.
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System iN. Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001.
- Morgan, B. The Human Complement System in Health and Disease. Ann. Rheum. Dis. 1998, 57, 581.
- Cho, H. Complement regulation: Physiology and disease relevance. Korean J. Pediatr. 2015, 58, 239–244.
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257.
- Dunkelberger, J.R.; Song, W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50.
- Gaboriaud, C.; Thielens, N.M.; Gregory, L.A.; Rossi, V.; Fontecilla-Camps, J.C.; Arlaud, G.J. Structure and activation of the C1 complex of complement: Unraveling the puzzle. Trends Immunol. 2004, 25, 368–373.
- Wallis, R.; Mitchell, D.A.; Schmid, R.; Schwaeble, W.J.; Keeble, A.H. Paths reunited: Initiation of the classical and lectin pathways of complement activation. Immunobiology 2010, 215, 1–11.
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344.
- Medzhitov, R.; Janeway, C.A., Jr. Decoding the patterns of self and nonself by the innate immune system. Science 2002, 296, 298–300.
- Harmat, V.; Gal, P.; Kardos, J.; Szilagyi, K.; Ambrus, G.; Vegh, B.; Naray-Szabo, G.; Zavodszky, P. The structure of MBL-associated serine protease-2 reveals that identical substrate specificities of C1s and MASP-2 are realized through different sets of enzyme-substrate interactions. J. Mol. Biol. 2004, 342, 1533–1546.
- Gal, P.; Barna, L.; Kocsis, A.; Zavodszky, P. Serine proteases of the classical and lectin pathways: Similarities and differences. Immunobiology 2007, 212, 267–277.
- Gupta, P.; Tripathy, A.S. Alternative pathway of complement activation has a beneficial role against Chandipura virus infection. Med. Microbiol. Immunol. 2020, 209, 109–124.
- Walport, M.J. Complement. Second of two parts. N. Engl. J. Med. 2001, 344, 1140–1144.
- Hourcade, D.E. Properdin and complement activation: A fresh perspective. Curr. Drug. Targets 2008, 9, 158–164.
- Harboe, M.; Mollnes, T.E. The alternative complement pathway revisited. J. Cell Mol. Med. 2008, 12, 1074–1084.
- Muller-Eberhard, H.J. The killer molecule of complement. J. Investig. Dermatol. 1985, 85, 47s–52s.
- Wetsel, R.A.; Kildsgaard, J.; Haviland, D.L. Complement anaphylatoxins (C3a, C4a, C5a) and their receptors (C3aR, C5aR/CD88) as therapeutic targets in inflammation. In Therapeutic Interventions in the Complement System. Contemporary Immunology; Lambris, J.D., Holers, M., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 113–153.
- Wang, H.; Ricklin, D.; Lambris, J.D. Complement-activation fragment C4a mediates effector functions by binding as untethered agonist to protease-activated receptors 1 and 4. Proc. Natl. Acad. Sci. USA 2017, 114, 10948–10953.
- Williams, T.J. Vascular permeability changes induced by complement-derived peptides. Agents Actions 1983, 13, 451–455.
- Takabayashi, T.; Vannier, E.; Clark, B.D.; Margolis, N.H.; Dinarello, C.A.; Burke, J.F.; Gelfand, J.A. A new biologic role for C3a and C3a desArg: Regulation of TNF-alpha and IL-1 beta synthesis. J. Immunol. 1996, 156, 3455–3460.
- Monsinjon, T.; Gasque, P.; Chan, P.; Ischenko, A.; Brady, J.J.; Fontaine, M.C. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003, 17, 1003–1014.
- Atkinson, J.P.; Du Clos, T.W.; Mold, C.; Kulkarni, H.; Hourcade, D.; Wu, X. The Human Complement System: Basic Concepts and Clinical Relevance. Capture 21; Elsevier: Amsterdam, The Netherlands, 2019.
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740.
- Liszewski, M.K.; Farries, T.C.; Lublin, D.M.; Rooney, I.A.; Atkinson, J.P. Control of the complement system. Adv. Immunol. 1996, 61, 201–283.
- Giannakis, E.; Jokiranta, T.S.; Male, D.A.; Ranganathan, S.; Ormsby, R.J.; Fischetti, V.A.; Mold, C.; Gordon, D.L. A common site within factor H SCR 7 responsible for binding heparin, C-reactive protein and streptococcal M protein. Eur. J. Immunol. 2003, 33, 962–969.
- Manuelian, T.; Hellwage, J.; Meri, S.; Caprioli, J.; Noris, M.; Heinen, S.; Jozsi, M.; Neumann, H.P.; Remuzzi, G.; Zipfel, P.F. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J. Clin. Investig. 2003, 111, 1181–1190.
- Morgan, H.P.; Schmidt, C.Q.; Guariento, M.; Blaum, B.S.; Gillespie, D.; Herbert, A.P.; Kavanagh, D.; Mertens, H.D.; Svergun, D.I.; Johansson, C.M.; et al. Structural basis for engagement by complement factor H of C3b on a self surface. Nat. Struct. Mol. Biol. 2011, 18, 463–470.
- Kim, D.D.; Song, W.C. Membrane complement regulatory proteins. Clin. Immunol. 2006, 118, 127–136.
- Kemper, C.; Atkinson, J.P.; Hourcade, D.E. Properdin: Emerging roles of a pattern-recognition molecule. Annu. Rev. Immunol. 2010, 28, 131–155.
- Agarwal, S.; Ferreira, V.P.; Cortes, C.; Pangburn, M.K.; Rice, P.A.; Ram, S. An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae. J. Immunol. 2010, 185, 507–516.
- Carroll, M.C. The complement system in B cell regulation. Mol. Immunol. 2004, 41, 141–146.
- Fang, Y.; Xu, C.; Fu, Y.X.; Holers, V.M.; Molina, H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J. Immunol. 1998, 160, 5273–5279.
- Carter, R.H.; Fearon, D.T. CD19: Lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 1992, 256, 105–107.
- Barrington, R.A.; Zhang, M.; Zhong, X.; Jonsson, H.; Holodick, N.; Cherukuri, A.; Pierce, S.K.; Rothstein, T.L.; Carroll, M.C. CD21/CD19 coreceptor signaling promotes B cell survival during primary immune responses. J. Immunol. 2005, 175, 2859–2867.
- Fischer, M.B.; Goerg, S.; Shen, L.; Prodeus, A.P.; Goodnow, C.C.; Kelsoe, G.; Carroll, M.C. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science 1998, 280, 582–585.
- Fischer, W.H.; Hugli, T.E. Regulation of B cell functions by C3a and C3a(desArg): Suppression of TNF-alpha, IL-6, and the polyclonal immune response. J. Immunol. 1997, 159, 4279–4286.
- Ottonello, L.; Corcione, A.; Tortolina, G.; Airoldi, I.; Albesiano, E.; Favre, A.; D’Agostino, R.; Malavasi, F.; Pistoia, V.; Dallegri, F. rC5a directs the in vitro migration of human memory and naive tonsillar B lymphocytes: Implications for B cell trafficking in secondary lymphoid tissues. J. Immunol. 1999, 162, 6510–6517.
- Longhi, M.P.; Harris, C.L.; Morgan, B.P.; Gallimore, A. Holding T cells in check—A new role for complement regulators? Trends Immunol. 2006, 27, 102–108.
- Le Friec, G.; Kemper, C. Complement: Coming full circle. Arch. Immunol. Ther. Exp. 2009, 57, 393–407.
- Liszewski, M.K.; Kolev, M.; Le Friec, G.; Leung, M.; Bertram, P.G.; Fara, A.F.; Subias, M.; Pickering, M.C.; Drouet, C.; Meri, S.; et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 2013, 39, 1143–1157.
- Elvington, M.; Liszewski, M.K.; Bertram, P.; Kulkarni, H.S.; Atkinson, J.P. A C3(H20) recycling pathway is a component of the intracellular complement system. J. Clin. Investig. 2017, 127, 970–981.
- Ghannam, A.; Fauquert, J.L.; Thomas, C.; Kemper, C.; Drouet, C. Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Mol. Immunol. 2014, 58, 98–107.
- Heeger, P.S.; Lalli, P.N.; Lin, F.; Valujskikh, A.; Liu, J.; Muqim, N.; Xu, Y.; Medof, M.E. Decay-accelerating factor modulates induction of T cell immunity. J. Exp. Med. 2005, 201, 1523–1530.
- Strainic, M.G.; Liu, J.; Huang, D.; An, F.; Lalli, P.N.; Muqim, N.; Shapiro, V.S.; Dubyak, G.R.; Heeger, P.S.; Medof, M.E. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 2008, 28, 425–435.
- Strainic, M.G.; Shevach, E.M.; An, F.; Lin, F.; Medof, M.E. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat. Immunol. 2013, 14, 162–171.
- Mellors, J.; Tipton, T.; Longet, S.; Carroll, M. Viral Evasion of the Complement System and Its Importance for Vaccines and Therapeutics. Front. Immunol. 2020, 11, 1450.
- Brudner, M.; Karpel, M.; Lear, C.; Chen, L.; Yantosca, L.M.; Scully, C.; Sarraju, A.; Sokolovska, A.; Zariffard, M.R.; Eisen, D.P.; et al. Lectin-dependent enhancement of Ebola virus infection via soluble and transmembrane C-type lectin receptors. PLoS ONE 2013, 8, e60838.
- Ji, X.; Olinger, G.G.; Aris, S.; Chen, Y.; Gewurz, H.; Spear, G.T. Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J. Gen. Virol. 2005, 86, 2535–2542.
- Ezekowitz, R.A.; Kuhlman, M.; Groopman, J.E.; Byrn, R.A. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J. Exp. Med. 1989, 169, 185–196.
- Ying, H.; Ji, X.; Hart, M.L.; Gupta, K.; Saifuddin, M.; Zariffard, M.R.; Spear, G.T. Interaction of mannose-binding lectin with HIV type 1 is sufficient for virus opsonization but not neutralization. AIDS Res. Hum. Retrovir. 2004, 20, 327–335.
- Crisci, E.; Ellegard, R.; Nystrom, S.; Rondahl, E.; Serrander, L.; Bergstrom, T.; Sjowall, C.; Eriksson, K.; Larsson, M. Complement Opsonization Promotes Herpes Simplex Virus 2 Infection of Human Dendritic Cells. J. Virol. 2016, 90, 4939–4950.
- Fuchs, A.; Lin, T.Y.; Beasley, D.W.; Stover, C.M.; Schwaeble, W.J.; Pierson, T.C.; Diamond, M.S. Direct complement restriction of flavivirus infection requires glycan recognition by mannose-binding lectin. Cell Host Microbe 2010, 8, 186–195.
- Johnson, J.B.; Capraro, G.A.; Parks, G.D. Differential mechanisms of complement-mediated neutralization of the closely related paramyxoviruses simian virus 5 and mumps virus. Virology 2008, 376, 112–123.
- Jayasekera, J.P.; Moseman, E.A.; Carroll, M.C. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 2007, 81, 3487–3494.
- Tam, J.C.; Bidgood, S.R.; McEwan, W.A.; James, L.C. Intracellular sensing of complement C3 activates cell autonomous immunity. Science 2014, 345, 1256070.
- Shaw, M.L.; Stone, K.L.; Colangelo, C.M.; Gulcicek, E.E.; Palese, P. Cellular proteins in influenza virus particles. PLoS Pathog. 2008, 4, e1000085.
- Rattan, A.; Pawar, S.D.; Nawadkar, R.; Kulkarni, N.; Lal, G.; Mullick, J.; Sahu, A. Synergy between the classical and alternative pathways of complement is essential for conferring effective protection against the pandemic influenza A(H1N1) 2009 virus infection. PLoS Pathog. 2017, 13, e1006248.
- Zhang, J.; Li, G.; Liu, X.; Wang, Z.; Liu, W.; Ye, X. Influenza A virus M1 blocks the classical complement pathway through interacting with C1qA. J. Gen. Virol. 2009, 90, 2751–2758.
- Kumar, N.A.; Kunnakkadan, U.; Thomas, S.; Johnson, J.B. In the Crosshairs: RNA Viruses OR Complement? Front. Immunol. 2020, 11, 573583.
- Shan, C.; Zhang, S.; Cui, W.; You, X.; Kong, G.; Du, Y.; Qiu, L.; Ye, L.; Zhang, X. Hepatitis B virus X protein activates CD59 involving DNA binding and let-7i in protection of hepatoma and hepatic cells from complement attack. Carcinogenesis 2011, 32, 1190–1197.
- Feng, G.; Li, J.; Zheng, M.; Yang, Z.; Liu, Y.; Zhang, S.; Ye, L.; Zhang, W.; Zhang, X. Hepatitis B virus X protein up-regulates C4b-binding protein alpha through activating transcription factor Sp1 in protection of hepatoma cells from complement attack. Oncotarget 2016, 7, 28013–28026.
- Avirutnan, P.; Hauhart, R.E.; Somnuke, P.; Blom, A.M.; Diamond, M.S.; Atkinson, J.P. Binding of flavivirus nonstructural protein NS1 to C4b binding protein modulates complement activation. J. Immunol. 2011, 187, 424–433.
- Thiemmeca, S.; Tamdet, C.; Punyadee, N.; Prommool, T.; Songjaeng, A.; Noisakran, S.; Puttikhunt, C.; Atkinson, J.P.; Diamond, M.S.; Ponlawat, A.; et al. Secreted NS1 Protects Dengue Virus from Mannose-Binding Lectin-Mediated Neutralization. J. Immunol. 2016, 197, 4053–4065.
- Kurosu, T.; Chaichana, P.; Yamate, M.; Anantapreecha, S.; Ikuta, K. Secreted complement regulatory protein clusterin interacts with dengue virus nonstructural protein 1. Biochem. Biophys. Res. Commun. 2007, 362, 1051–1056.
- Conde, J.N.; da Silva, E.M.; Allonso, D.; Coelho, D.R.; Andrade, I.D.S.; de Medeiros, L.N.; Menezes, J.L.; Barbosa, A.S.; Mohana-Borges, R. Inhibition of the Membrane Attack Complex by Dengue Virus NS1 through Interaction with Vitronectin and Terminal Complement Proteins. J. Virol. 2016, 90, 9570–9581.
- Malekshahi, Z.; Bernklau, S.; Schiela, B.; Koske, I.; Banki, Z.; Stiasny, K.; Harris, C.L.; Wurzner, R.; Stoiber, H. Incorporation of CD55 into the Zika Viral Envelope Contributes to Its Stability against Human Complement. Viruses 2021, 13, 510.
- Chung, K.M.; Liszewski, M.K.; Nybakken, G.; Davis, A.E.; Townsend, R.R.; Fremont, D.H.; Atkinson, J.P.; Diamond, M.S. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc. Natl. Acad. Sci. USA 2006, 103, 19111–19116.
