Lipids from algae have been scarcely applied to modulate skin diseases, but they are well known antioxidant and anti-inflammatory agents. They have shown scavenging activities and can modulate redox homeostasis enzymes. They can also downmodulate key inflammatory signaling pathways and transcription factors such as NF-κB, decreasing the expression of pro-inflammatory mediators. Thus, the exploitation of algae lipids as therapeutical agents for the treatment of inflammatory skin diseases is highly attractive.
- skin diseases
- inflammation
- oxidative stress
- lipidomics
- bioactive lipids
- anti-inflammatory
- antioxidant
- macroalgae
- microalgae
1. Introduction
2. Algae Lipids with Antioxidant Activity
| Studies | Mechanism | Assay | Identified Lipids | Algae Species | Ref. |
|---|---|---|---|---|---|
| in chemico | Free radical scavenging | ABTS, DPPH, hydroxyl radical, superoxide anion | Polar lipids, neutral lipids, FAME | Macroalgae: Bifurcaria bifurcata, Codium tomentosum, Fucus vesiculosus, Gracilaria gracilis Grateloupia turuturu, Palmaria palmata, Porphyra dioica Sargassum muticum, Solieria chordalis, Ulva rigida Microalgae: Chlorella vulgaris, Chlorococcum amblystomatis, Nannochloropsis oceanica, Phaeodactylum tricornutum, Scenedesmus intermedius Scenedesmus obliquus, Spirulina sp., Tetraselmis chui |
[19][20][21][22] |
| in vitro | Detoxify intracellular ROS |
Increased the expression of Nrf2 in irradiated HaCat cells Upregulate target antioxidant enzymes Cu/Zn SOD, CAT, and HO-1 |
Crude ethanolic extract | Macroalga: Carpomitra costata | [23] |
| Free radical scavenging | Superoxide generation on peritoneal leukocytes | Sulfoquinovosylacylglycerols | Microalgae: Porphyridium cruentum | [24] | |
| Inhibition of ROS | Photoprotective against UVB in NHDF | Crude ethyl acetate extract | Microalga: Ettlia sp. YC001 | [25] | |
| Enzyme/protein expression | Downregulation of expression of MMPs | Crude ethanolic extract | Microalga: Arthrospira platensis | [26] | |
| Enzyme/protein expression | Downregulation of expression of MMPs, IL-6 and TGF-1 in human dermal fibroblast Modulate MAPK in irradiated HaCat cells |
Fucosterol | Macroalga: Sargassum fusiforme | [27][28] |
The pathophysiology of inflammatory skin diseases is associated with unregulated elevated levels of ROS and the activity of enzymes and proteins involved in the regulation of oxidative stress [29]. In cells, mitochondria metabolize oxygen, producing ROS. During the oxidative phosphorylation in mitochondria, oxygen is converted to O2•−, which can be transformed in H2O2 by superoxide dismutase, and then to water by glutathione peroxidase (GPX) or peroxiredoxin III (PRX III) radical [30]. Under normal conditions, the mitochondria ROS production is balanced by the production of a variety of antioxidants. However, oxidative stress occurs when there is an imbalance between ROS and antioxidants production. An imbalance in ROS production leads to redox signaling from cellular organelles, causing mitochondrial damage and dysfunction in several conditions [31]. However, the application of algae lipids to prevent mitochondrial dysfunction and modulate the oxidative status is little understood and requires in-depth study to understand the mechanisms underlying this potential antioxidant role. The use of crude extracts from algae may reduce ROS levels induced by UVB and impair the expression of MMPs and thymine dimers formation due to UVB exposure in skin cells [26]. These studies were performed using complex crude extracts rich in lipids and not with isolated lipids or fractions. This hinders the understanding of the mechanisms of action of algal lipids as antioxidants, and more work is needed to determine the potential protective role of algal lipids in skin diseases. A better understanding of this antioxidant action is needed, for example, there is a lack of knowledge about the impact of specific lipid classes or lipid molecules in the enzymes and proteins involved in the regulation of oxidative stress, such as metalloproteinases, HO-1, catalase, or superoxide dismutase.
3. Algae Lipids with Anti-Inflammatory Activity
| Studies | Action | Model | Identified Lipids | Algae Species | Ref. |
|---|---|---|---|---|---|
| In chemico | COX-2 inhibition | COX-2 kit assay | Polar lipids | Macroalgae: Codium tomentosum, Fucus vesiculosus Gracilaria gracilis, Palmaria palmata, Porphyra dioica, Ulva rigida, Microalgae: Chlorella vulgaris, Chlorococcum amblystomatis, Gloeothece sp., Skeletonema sp., Tetraselmis sp. mutants |
[19][32][33][34][35] |
| In vitro | NO inhibition | Raw 264.7 | Polar and non-polar lipids; PC, PG, DGDG, DGTS, MGDG, MGMG, SQDG classes; Free and ethyl esterified DGLA |
Macroalgae: Chondrus crispus, Lobophora sp.Palmaria palmata, Microalgae: Chlorella sorokiniana Lobosphaera incisa, Nannochloropsis granulata, Tetraselmis chui, |
[36][37][38][39][40][41][42][43] |
| Decrease in PGE2 Downregulation of COX-2 | Raw 264.7; White blood cells; Epidermal cells |
Crude ethanolic extracts; lipid extracts rich in PC; free and ethyl esterified DGLA |
Macroalgae: Laminaria ochroleuca Microalgae: Chlorella vulgaris, Chloromonas reticulata, Lobosphaera incisa Micractinium sp., Phaeodactylum tricornutum, |
[41][44][45][46][47][48] | |
| Downregulation of mRNA expression of pro-inflammatory cytokines Downregulation of cytokines levels: TNF-α, IL-6, IL-1α, and IL-1β |
THP-1; PBMC; Epidermal cells; HaCaT cells |
Crude ethanolic extracts; lipid extracts; lipid extracts rich in MGDG, DGDG and SQDG; Lipid extracts rich in PC; LPC(16:0); oxylipins; ergosterol and 7-dehydroporiferasterol; free and ethyl esterified DGLA |
Macroalgae: Chondrus crispus, Laminaria ochroleuca, Palmaria palmata, Porphyra dioica, Prasiola japonica Microalgae: Aurantiochytrium mangrovei, Chlamydomonas debaryana, Chlorella vulgaris, Chloromonas reticulata, Cylindrotheca closterium, Dunaliella tertiolecta, Micratinium sp., Nannochloropsis gaditana, Nitzschia palea, Phaeodactylum tricornutum, Lobosphaera incisa Spirulina maxima, Pavlova lutheri, Tetraselmis suecica, |
[23][41][44][45][46][47][49][50][51][52][53][54][55][56][48][57][58] | |
| Inhibition of pro-inflammatory signaling pathways mediated by TLR and NF-κB | THP-1 | Lipid extracts rich in MGDG, DGDG, and SQDG | Macroalgae: Chondrus crispus, Palmaria palmata, Porphyra dioica Microalgae: Pavlova lutheri |
[49] | |
| In vivo | Attenuation of ear oedema | PLA2 kit assay; Mice with ear oedema; DNFB-induced in naive C57BL/6 mice |
MMHDA; Lipid extracts rich in PC; MGDG, DGDG, and SQDG fractions |
Macroalgae: Ishige okamurae, Laminaria ochroleuca Microalgae: ETS-05 cyanobacterium. |
[48][59][60] |
| Neutrophil gathering in the wound region | Wounded zebrafish model | Glycolipids rich in γ-linolenic acid | Microlagae: Spirulina platensis | [61] | |
| Inhibition of pro-inflammatory cytokines production: TNF-α, IL-6, IL-8, IFN- γ, IL-1β, IL-17 | db/db and CD1 mice model of diabetes mellitus; TNBS-induced colitis rats; BALB/c mice skin; TPA-induced hyperplasia murine model |
Crude ethanolic extract; omega-3 fatty acids; oxylipins; MGDG cream |
Macroalgae: Sargassum cristaefolium Microalgae: Chlamydomonas debaryana, Isochrysis galbana |
[62][63][64][65] | |
| Downregulation of iNOS and COX-2, and decrease in NO and PGE2 production | TNBS-induced colitis rat; BALB/c mice skin; TPA-induced hyperplasia murine model |
Crude ethanolic extract; oxylipins; MGDG cream | Macroalgae: Sargassum cristaefolium Microalgae: Chlamydomonas debaryana, Isochrysis galbana |
[63][64][65] |
It was demonstrated that methoxylated fatty acids (MMHDA) isolated from macroalga Ishige okamurae [60] and MGDG, DGDG, and SQDG fractions from microalga ETS-05 cyanobacterium [59] presented anti-inflammatory activity by reducing ear oedema (swelling) in a mouse model. The anti-inflammatory action of MMHDA has been associated with the inhibition of phospholipase A2 (PLA2), the enzyme responsible for the hydrolysis of the sn-2 position of membrane glycerophospholipids to liberate arachidonic acid (AA). The reduction in neutrophils was observed in the wound region of a zebrafish model when glycolipids rich in γ-linolenic acid from the microalga Spirulina platensis were used [61]. Extracts with omega-3 FA isolated from microalgae promoted the reduction of CD4+ T cells production of the pro-inflammatory mediators IFN-γ, TNF-α, and IL-4 and increased the secretion of IL-17A, IL-14, and TGF-β in a db/db and CD1 mouse model of diabetes Mellitus [62]. Downregulation of TNF-α was also observed, as well as decreased expression of iNOS and COX-2, when 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis rats were supplemented with oxylipins extracted from Chlamydomonas debaryana [63].
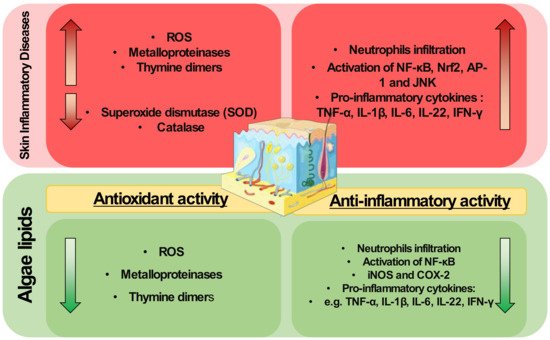
4. Concluding Remarks
This entry is adapted from the peer-reviewed paper 10.3390/metabo12020096
References
- Benson, H.A.E.; Watkinson, A.C. Transdermal and Topical Drug Delivery: Principles and Practice; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-0-470-45029-1.
- Choi, W.; Wolber, R.; Gerwat, W.; Mann, T.; Batzer, J.; Smuda, C.; Liu, H.; Kolbe, L.; Hearing, V.J. The Fibroblast-Derived Paracrine Factor Neuregulin-1 Has a Novel Role in Regulating the Constitutive Color and Melanocyte Function in Human Skin. J. Cell Sci. 2010, 123, 3102–3111.
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J. Role of Oxidative Stress and the Antioxidant Network in Cutaneous Carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335.
- Kruk, J.; Duchnik, E. Oxidative Stress and Skin Diseases: Possible Role of Physical Activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568.
- Albanesi, C.; Pastore, S. Pathobiology of Chronic Inflammatory Skin Diseases: Interplay Between Keratinocytes and Immune Cells as a Target for Anti-Inflammatory Drugs. Curr. Drug Metab. 2010, 11, 210–227.
- Rashigni, M.; Harris, J.E. Vitiligo Pathogenesis and Emerging Treatments. Dermatol. Clin. 2017, 35, 257–265.
- Lopez Carrera, Y.I.; Al Hammadi, A.; Huang, Y.H.; Llamado, L.J.; Mahgoub, E.; Tallman, A.M. Epidemiology, Diagnosis, and Treatment of Atopic Dermatitis in the Developing Countries of Asia, Africa, Latin America, and the Middle East: A Review. Dermatol. Ther. 2019, 9, 685–705.
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine Algae as Attractive Source to Skin Care. Free Radic. Res. 2017, 51, 555–567.
- Lee, J.H.; Lim, J.Y.; Jo, E.H.; Noh, H.M.; Park, S.; Park, M.C.; Kim, D.K. Chijabyukpi-Tang Inhibits Pro-Inflammatory Cytokines and Chemokines via the Nrf2/HO-1 Signaling Pathway in TNF-α/IFN-γ-Stimulated HaCaT Cells and Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice. Front. Pharmacol. 2020, 11, 1–11.
- Parvez, S.; Kang, M.; Chung, H.-S.; Cho, C.; Hong, M.-C.; Shin, M.-K.; Bae, H. Survey and Mechanism of Skin Depigmenting and Lightening Agents. Phytother. Res. 2006, 20, 921–934.
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484.
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2020, 18, 17.
- Guesmi, A.; Boumaiza, M.; Boudabous, A. Microbiological Quality and Safety of Commercialized Thalassotherapy Products Based on Marine Mud and Algae Extracts in Tunisia. Arch. Microbiol. 2020, 202, 2437–2451.
- Pulz, O.; Gross, W. Valuable Products from Biotechnology of Microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648.
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52.
- Choo, W.T.; Teoh, M.L.; Phang, S.M.; Convey, P.; Yap, W.H.; Goh, B.H.; Beardall, J. Microalgae as Potential Anti-Inflammatory Natural Product Against Human Inflammatory Skin Diseases. Front. Pharmacol. 2020, 11, 1–11.
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945.
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160.
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications. Molecules 2020, 25, 3883.
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as Sustainable Bio-Factories of Healthy Lipids: Evaluating Fatty Acid Content and Antioxidant Activity. Mar. Drugs 2021, 19, 357.
- Terme, N.; Boulho, R.; Kucma, J.-P.; Bourgougnon, N.; Bedoux, G. Radical Scavenging Activity of Lipids from Seaweeds Isolated by Solid-Liquid Extraction and Supercritical Fluids. OCL 2018, 25, D505.
- Davoodbasha, M.; Edachery, B.; Nooruddin, T.; Lee, S.-Y.; Kim, J.-W. An Evidence of C16 Fatty Acid Methyl Esters Extracted from Microalga for Effective Antimicrobial and Antioxidant Property. Microb. Pathog. 2018, 115, 233–238.
- Zheng, J.; Hewage, S.R.K.M.; Piao, M.J.; Kang, K.A.; Han, X.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H.; Ko, C.S.; et al. Photoprotective Effect of Carpomitra costata Extract against Ultraviolet B-Induced Oxidative Damage in Human Keratinocytes. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 11–28.
- Bergé, J.P.; Debiton, E.; Dumay, J.; Durand, P.; Barthomeuf, C. In vitro Anti-Inflammatory and Anti-Proliferative Activity of Sulfolipids from the Red Alga Porphyridium cruentum. J. Agric. Food Chem. 2002, 50, 6227–6232.
- Lee, J.-J.; An, S.; Kim, K.B.; Heo, J.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S.; Bae, S. Extract of Ettlia sp. YC001 Exerts Photoprotective Effects against UVB Irradiation in Normal Human Dermal Fibroblasts. J. Microbiol. Biotechnol. 2016, 26, 775–783.
- Lee, J.-J.; Kim, K.B.; Heo, J.; Cho, D.-H.; Kim, H.-S.; Han, S.H.; Ahn, K.J.; An, I.-S.; An, S.; Bae, S. Protective Effect of Arthrospira platensis Extracts against Ultraviolet B-Induced Cellular Senescence through Inhibition of DNA Damage and Matrix Metalloproteinase-1 Expression in Human Dermal Fibroblasts. J. Photochem. Photobiol. B Biol. 2017, 173, 196–203.
- Hwang, E.; Park, S.-Y.; Sun, Z.; Shin, H.-S.; Lee, D.-G.; Yi, T.H. The Protective Effects of Fucosterol against Skin Damage in UVB-Irradiated Human Dermal Fibroblasts. Mar. Biotechnol. 2014, 16, 361–370.
- Kim, M.-S.; Oh, G.-H.; Kim, M.-J.; Hwang, J.-K. Fucosterol Inhibits Matrix Metalloproteinase Expression and Promotes Type-1 Procollagen Production in UVB-Induced HaCaT Cells. Photochem. Photobiol. 2013, 89, 911–918.
- Marzano, A.V.; Ortega-Loayza, A.G.; Heath, M.; Morse, D.; Genovese, G.; Cugno, M. Mechanisms of Inflammation in Neutrophil-Mediated Skin Diseases. Front. Immunol. 2019, 10, 1059.
- Green, K.; Brand, M.D.; Murphy, M.P. Prevention of Mitochondrial Oxidative Damage as a Therapeutic Strategy in Diabetes. Diabetes 2004, 53, S110–S118.
- James, A.M.; Murphy, M.P. How Mitochondrial Damage Affects Cell Function. J. Biomed. Sci. 2002, 9, 475–487.
- da Costa, E.; Amaro, H.M.; Melo, T.; Guedes, A.C.; Domingues, M.R. Screening for Polar Lipids, Antioxidant, and Anti-Inflammatory Activities of Gloeothece sp. Lipid Extracts Pursuing New Phytochemicals from Cyanobacteria. J. Appl. Phycol. 2020, 32, 3015–3030.
- Conde, T.A.; Couto, D.; Melo, T.; Costa, M.; Silva, J.; Domingues, M.R.; Domingues, P. Polar Lipidomic Profile Shows Chlorococcum amblystomatis as a Promising Source of Value-Added Lipids. Sci. Rep. 2021, 11, 4355.
- Couto, D.; Melo, T.; Conde, T.A.; Costa, M.; Silva, J.; Domingues, M.R.M.; Domingues, P. Chemoplasticity of the Polar Lipid Profile of the Microalgae Chlorella vulgaris Grown under Heterotrophic and Autotrophic Conditions. Algal Res. 2020, 53, 102128.
- Cardoso, C.; Pereira, H.; Franca, J.; Matos, J.; Monteiro, I.; Pousão-Ferreira, P.; Gomes, A.; Barreira, L.; Varela, J.; Neng, N.; et al. Lipid Composition and Some Bioactivities of 3 Newly Isolated Microalgae (Tetraselmis sp. IMP3, Tetraselmis sp. CTP4, and Skeletonema sp.). Aquac. Int. 2020, 28, 711–727.
- Pham, T.H.; Nguyen, V.T.A.; Do, T.T.T.; Do, A.D.; Dam, D.T.; Tran, T.T.V.; Pham, Q.L.; Le, T.T. Lipidomics and Anti-Inflammation Activity of Brown Algae, Lobophora sp., in Vietnam. J. Chem. 2020, 2020, 8829054.
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T. Lipids Isolated from the Cultivated Red Alga Chondrus crispus Inhibit Nitric Oxide Production. J. Appl. Phycol. 2014, 26, 1565–1571.
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A.T. Polar Lipids from the Marine Macroalga Palmaria palmata Inhibit Lipopolysaccharide-Induced Nitric Oxide Production in RAW264.7 Macrophage Cells. Phytochemistry 2014, 101, 101–108.
- Banskota, A.H.; Gallant, P.; Stefanova, R.; Melanson, R.; Oleary, S.J.B. Monogalactosyldiacylglycerols, Potent Nitric Oxide Inhibitors from the Marine Microalga Tetraselmis chui. Nat. Prod. Res. 2013, 27, 1084–1090.
- Banskota, A.H.; Stefanova, R.; Gallant, P.; McGinn, P.J. Mono- and Digalactosyldiacylglycerols: Potent Nitric Oxide Inhibitors from the Marine Microalga Nannochloropsis granulata. J. Appl. Phycol. 2013, 25, 349–357.
- Novichkova, E.; Chumin, K.; Eretz-Kdosha, N.; Boussiba, S.; Gopas, J.; Cohen, G.; Khozin-Goldberg, I. DGLA from the Microalga Lobosphaera incsa P127 Modulates Inflammatory Response, Inhibits INOS Expression and Alleviates NO Secretion in RAW264.7 Murine Macrophages. Nutrients 2020, 12, 2892.
- Banskota, A.H.; Stefanova, R.; Sperker, S.; McGinn, P.J. New Diacylglyceryltrimethylhomoserines from the Marine Microalga Nannochloropsis granulata and Their Nitric Oxide Inhibitory Activity. J. Appl. Phycol. 2013, 25, 1513–1521.
- Banskota, A.H.; Stefanova, R.; Gallant, P.; Osborne, J.A.; Melanson, R.; O’Leary, S.J.B. Nitric Oxide Inhibitory Activity of Monogalactosylmonoacylglycerols from a Freshwater Microalgae Chlorella sorokiniana. Nat. Prod. Res. 2013, 27, 1028–1031.
- Suh, S.S.; Hong, J.M.; Kim, E.J.; Jung, S.W.; Kim, S.M.; Kim, J.E.; Kim, I.C.; Kim, S. Anti-Inflammation and Anti-Cancer Activity of Ethanol Extract of Antarctic Freshwater Microalga, Micractinium sp. Int. J. Med Sci. 2018, 15, 929–936.
- Abu-Serie, M.M.; Habashy, N.H.; Attia, W.E. In vitro Evaluation of the Synergistic Antioxidant and Anti-Inflammatory Activities of the Combined Extracts from Malaysian Ganoderma lucidum and Egyptian Chlorella vulgaris. BMC Complement. Altern. Med. 2018, 18, 154.
- Neumann, U.; Louis, S.; Gille, A.; Derwenskus, F.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Anti-Inflammatory Effects of Phaeodactylum tricornutum Extracts on Human Blood Mononuclear Cells and Murine Macrophages. J. Appl. Phycol. 2018, 30, 2837–2846.
- Suh, S.S.; Hong, J.M.; Kim, E.J.; Jung, S.W.; Chae, H.; Kim, J.E.; Kim, J.H.; Kim, I.C.; Kim, S. Antarctic Freshwater Microalga, Chloromonas reticulata, Suppresses Inflammation and Carcinogenesis. Int. J. Med. Sci. 2019, 16, 189–197.
- Bonneville, M.; Saint-Mezard, P.; Benetiere, J.; Hennino, A.; Pernet, I.; Denis, A.; Nicolas, J. Laminaria ochroleuca Extract Reduces Skin Inflammation. J. Eur. Acad. Dermatol. Venerol. 2007, 21, 1124–1125.
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The Anti-Inflammatory Effect of Algae-Derived Lipid Extracts on Lipopolysaccharide (LPS)-Stimulated Human THP-1 Macrophages. Mar. Drugs 2015, 13, 5402–5424.
- Choi, E.; Yi, Y.-S.; Lee, J.; Park, S.H.; Kim, S.; Hossain, M.A.; Jang, S.; Choi, Y.I.; Park, K.J.; Kim, D.S.; et al. Anti-Apoptotic and Anti-Inflammatory Activities of Edible Fresh Water Algae Prasiola japonica in UVB-Irradiated Skin Keratinocytes. Am. J. Chin. Med. 2019, 47, 1853–1868.
- Lakshmegowda, S.B.; Rajesh, S.K.; Kandikattu, H.K.; Nallamuthu, I.; Khanum, F. In vitro and in vivo Studies on Hexane Fraction of Nitzschia palea, a Freshwater Diatom for Oxidative Damage Protective and Anti-Inflammatory Response. Rev. Bras. Farmacogn. 2020, 30, 189–201.
- Sibi, G.; Rabina, S. Inhibition of Pro-Inflammatory Mediators and Cytokines by Chlorella vulgaris Extracts. Pharmacogn. Res. 2016, 8, 118–122.
- Jo, W.S.; Choi, Y.J.; Kim, H.J.; Nam, B.H.; Hong, S.H.; Lee, G.A.; Lee, S.W.; Seo, S.Y.; Jeong, M.H. Anti-Inflammatory Effect of Microalgal Extracts from Tetraselmis suecica. Food Sci. Biotechnol. 2010, 19, 1519–1528.
- Takahashi, S.; Sakamaki, M.; Ferdousi, F.; Yoshida, M.; Demura, M.; Watanabe, M.M.; Isoda, H. Ethanol Extract of Aurantiochytrium mangrovei 18W-13a Strain Possesses Anti-Inflammatory Effects on Murine Macrophage RAW264 Cells. Front. Physiol. 2018, 9, 1–10.
- Choi, W.Y.; Sim, J.H.; Lee, J.Y.; Kang, D.H.; Lee, H.Y. Increased Anti-Inflammatory Effects on LPS-Induced Microglia Cells by Spirulina maxima Extract from Ultrasonic Process. Appl. Sci. 2019, 9, 2144.
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E.H. Lysophosphatidylcholines and Chlorophyll-Derived Molecules from the Diatom Cylindrotheca closterium with Anti-Inflammatory Activity. Mar. Drugs 2020, 18, 166.
- De Los Reyes, C.; Ávila-Román, J.; Ortega, M.J.; De La Jara, A.; García-Mauriño, S.; Motilva, V.; Zubía, E. Oxylipins from the Microalgae Chlamydomonas debaryana and Nannochloropsis gaditana and Their Activity as TNF-α Inhibitors. Phytochemistry 2014, 102, 152–161.
- Caroprese, M.; Albenzio, M.; Ciliberti, M.G.; Francavilla, M.; Sevi, A. A Mixture of Phytosterols from Dunaliella tertiolecta Affects Proliferation of Peripheral Blood Mononuclear Cells and Cytokine Production in Sheep. Vet. Immunol. Immunopathol. 2012, 150, 27–35.
- Bruno, A.; Rossi, C.; Marcolongo, G.; Di Lena, A.; Venzo, A.; Berrie, C.P.; Corda, D. Selective in vivo Anti-Inflammatory Action of the Galactolipid Monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168.
- Cho, J.-Y.; Gyawali, Y.P.; Ahn, S.-H.; Khan, M.N.A.; Kong, I.-S.; Hong, Y.-K. A Methoxylated Fatty Acid Isolated from the Brown Seaweed Ishige okamurae Inhibits Bacterial Phospholipase A2. Phytother. Res. 2008, 22, 1070–1074.
- Yang, X.; Li, Y.; Li, Y.; Ye, D.; Yuan, L.; Sun, Y.; Han, D.; Hu, Q. Solid Matrix-Supported Supercritical CO2 Enhances Extraction of γ-Linolenic Acid from the Cyanobacterium Arthrospira (Spirulina) platensis and Bioactivity Evaluation of the Molecule in Zebrafish. Mar. Drugs 2019, 17, 203.
- Gutiérrez-Pliego, L.; Martínez-Carrillo, B.; Reséndiz-Albor, A.; Arciniega-Martínez, I.; Escoto-Herrera, J.; Rosales-Gómez, C.; Valdés-Ramos, R. Effect of Supplementation with n-3 Fatty Acids Extracted from Microalgae on Inflammation Biomarkers from Two Different Strains of Mice. J. Lipids 2018, 2018, 4765358.
- Avila-Román, J.; Talero, E.; Alcaide, A.; de Los Reyes, C.; Zubía, E.; García-Mauriño, S.; Motilva, V. Preventive Effect of the Microalga Chlamydomonas debaryana on the Acute Phase of Experimental Colitis in Rats. Br. J. Nutr. 2014, 112, 1055–1064.
- Prasedya, E.S.; Martyasari, N.W.R.; Abidin, A.S.; Pebriani, S.A.; Ilhami, B.T.K.; Frediansyah, A.; Sunarwidhi, A.L.; Widyastuti, S.; Sunarpi, H. Macroalgae Sargassum Cristaefolium Extract Inhibits Proinflammatory Cytokine Expression in BALB/C Mice. Scientifica 2020, 2020, 9769454.
- Rodríguez-Luna, A.; Talero, E.; Terencio, M.; González-Rodríguez, M.; Rabasco, A.; de los Reyes, C.; Motilva, V.; Ávila-Román, J. Topical Application of Glycolipids from Isochrysis galbana Prevents Epidermal Hyperplasia in Mice. Mar. Drugs 2017, 16, 2.
- Alhayaza, R.; Haque, E.; Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front. Chem. 2020, 8, 592688.
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772.
