Primary knee osteoarthritis (OA) continues to be a hard-to-control degenerative disease. Intra-articular corticosteroids are typically advised, but only for short-term pain alleviation, given that their benefits last only a few weeks. The efficacy of hyaluronic acid is controversial. When the aforesaid options fail, total knee arthroplasty is generally recommended as an efficacious treatment. However, it is costly and can involve medical and postoperative complications. Therefore, determining alternate safe and effective treatments for knee OA is paramount. Platelet-rich plasma (PRP) has lately been investigated for the treatment of knee OA.
- platelet-rich plasma
- knee
- osteoarthritis
- mechanisms of action
- efficacy
1. Introduction
2. Platelet-Rich Plasma’s Molecular Mechanisms of Action
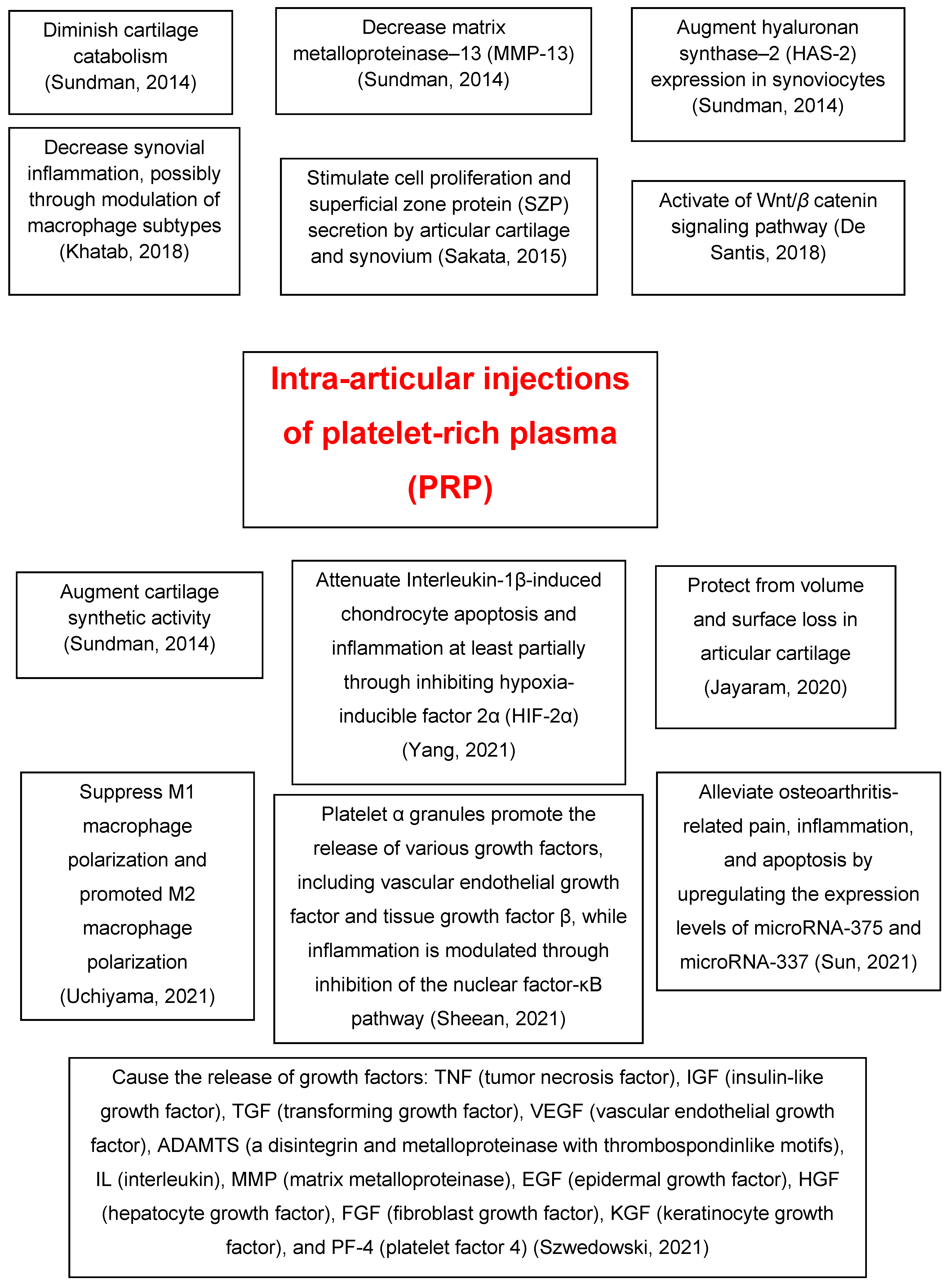
|
Authors |
Year |
Main Findings |
|---|---|---|
|
Sundman et al. [27] |
2014 |
PRP treatment decreases catabolism and matrix metalloproteinase-13 and increases hyaluronan synthase-2 expression in synoviocytes and cartilage synthetic activity. |
|
Sakata et al. [28] |
2015 |
PRP stimulates cell proliferation and superficial zone protein secretion by articular cartilage and synovium of the human knee joint. |
|
Khatab et al. [29] |
2018 |
Multiple PRP releasate injections reduce pain and synovial thickness, possibly through modulation of macrophage subtypes. |
|
De Santis et al. [30] |
2018 |
PRP therapy for OA exerts modulation on the Wnt/β catenin pathway that might be relevant in achieving its beneficial clinical effect. |
|
Liu et al. [31] |
2019 |
The therapeutic effects of exosomes derived from PRP on OA were similar or better compared with those of activated PRP in vitro or in vivo. |
|
Jayaram et al. [4] |
2020 |
The effects of PRP therapy on OA progression and disease-induced hyperalgesia might be leukocyte-dependent. |
|
Yang et al. [32] |
2021 |
PRP attenuates interleukin-1β, inducing chondrocyte apoptosis and inflammation at least partially through inhibiting hypoxia-inducible factor 2α. |
|
Sun et al. [33] |
2021 |
Micro-RNA (miR)-337 and miR-375 are involved in PRP-delayed OA progression by affecting inflammation and apoptosis. |
|
Sheean et al. [34] |
2021 |
Platelet α granules promote the release of various growth factors, including vascular endothelial growth factor and tissue growth factor β, and inflammation is modulated through inhibition of the nuclear factor-κB pathway. |
|
Uchiyama et al. [35] |
2021 |
The autologous protein solution leukocyte-rich PRP kit has a higher concentration of M1 and M2 macrophage-related factors. |
|
Szwedowski et al. [36] |
2021 |
Growth factors released in the OA knee joint after PRP injection: tumor necrosis factor, insulin-like growth factor, transforming growth factor, vascular endothelial growth factor, a disintegrin and metalloproteinase with thrombospondin motifs, interleukin, matrix metalloproteinase, epidermal growth factor, hepatocyte growth factor, fibroblast growth factor, keratinocyte growth factor, and platelet factor 4. |
PRP = platelet-rich plasma; OA = osteoarthritis.
3. Efficacy of Intra-Articular Platelet-Rich Plasma Injections in Knee Osteoarthritis
3.1. Placebo-Controlled Trials
3.2. Randomized Controlled Trials
3.3. Systematic Reviews and Meta-Analyses
3.4. Case Series
Table 2 summarizes the efficacy of intra-articular PRP injections in knee OA [37][38][39][40][41][42][43][44][45][46][47][48][49][50][51].
|
Authors |
Year |
Type of Study |
Main Findings |
|---|---|---|---|
|
Tucker et al. [37] |
2021 |
Single-blinded, randomized, placebo-controlled pilot study |
The PRP treatment group had less pain and stiffness and improved function scores than the placebo (saline) group |
|
Yurtbay et al. [38] |
2021 |
Randomized, double-blind, placebo-controlled clinical trial |
Compared with placebo (sodium saline), LR-PRP treatment was effective in the treatment of OA. Multiple doses of PRP increased the treatment efficacy and duration. Patients aged 51–65 years scored better at 6 months |
|
Bennell et al. [39] |
2021 |
Randomized, 2-group, placebo-controlled, participant-, injector-, and assessor-blinded clinical trial |
Among patients with symptomatic mild-to-moderate radiographic knee OA, intra-articular PRP injection, compared with injection of saline placebo, did not result in a significant difference in symptoms or joint structure at 12 months. |
|
Dório et al. [40] |
2021 |
Randomized, double-blind, placebo-controlled trial of 3 groups of patients: PRP, plasma, and saline. |
There were no differences among the 3 study groups at weeks 6 and 12. |
|
Kim et al. [41] |
2021 |
Systematic review and meta-analysis (level of evidence IV) |
Intra-articular PRP injection resulted in improvements above the minimal clinically important difference in terms of pain and function up to 12 months. |
|
Nie et al. [42] |
2021 |
Meta-analysis of randomized controlled clinical trials (level of evidence I) |
PRP injections were beneficial for pain alleviation and functional improvement in knee OA. |
|
Li et al. [43] |
2021 |
Literature review |
Compared with many other treatment methods, intra-articular injection of PRP proved to be safe and effective to improve the quality of life of patients with knee OA. |
|
Hong et al. [44] |
2021 |
Systematic review and meta-analysis |
Compared with placebo, PRP had a lower VAS score and higher IKDC subjective score at 6 months after treatment and a significantly lower WOMAC score during the follow-up period. |
|
Aiyer et al. [45] |
2021 |
Systematic review of clinical studies |
These authors recommended PRP for patients with early-stage OA (I or II) and who are aged younger than 65. |
|
Moton et al. [46] |
2021 |
Prospective case series |
PRP injections for treating OA (grade 1 to 3) proved to be successful in terms of improving functional outcomes and reducing pain intensity. |
|
Sun et al. [47] |
2021 |
Case series |
One injection of PRP improved pain and function for 6 months for patients with early knee OA. |
|
Bec et al. [48] |
2021 |
Case series (retrospective study) |
A single injection of pure PRP resulted in significant clinical improvement in the management of knee OA. |
|
Hegaze et al. [49] |
2021 |
Prospective case series |
Intra-articular injections gave significant pain and flexion improvement in patients with grades II, III, and IV OA, especially with multiple injections in the short-term follow-up. |
|
Rai et al. [50] |
2021 |
Case series |
PRP was a safe and effective therapy for early OA knees. It provided a significant clinical improvement in patients, with some loss of improvement with time. |
|
Jayaram et al. [51] |
2021 |
Case series |
LR-PRP demonstrated efficacy in meaningful end points for functional and patient-reported outcomes at early time points in patients with knee OA. |
PRP = platelet-rich plasma; LR-PRP = leukocyte rich PRP; OA = osteoarthritis; VAS = visual analog scale; IKDC = International Knee Documentation Committee; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031301
References
- Rodríguez-Merchán, E.C. Intra-articular injections of hyaluronic acid and other drugs in the knee joint. HSS J. 2013, 9, 180–182.
- Cohen, S.A.; Zhuang, T.; Xiao, M.; Michaud, J.B.; Amanatullah, D.F.; Kamal, R.N. Google trends analysis shows increasing public interest in platelet-rich plasma injections for hip and knee osteoarthritis. J. Arthroplasty 2021, 36, 3616–3622.
- Rodriguez-Merchan, E.C. Intraarticular injections of platelet-rich plasma (PRP) in the management of knee osteoarthritis. Arch. Bone Jt. Surg. 2013, 1, 5–8.
- Jayaram, P.; Liu, C.; Dawson, B.; Ketkar, S.; Patel, S.J.; Lee, B.H.; Grol, M.W. Leukocyte-dependent effects of platelet-rich plasma on cartilage loss and thermal hyperalgesia in a mouse model of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2020, 28, 1385–1393.
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; Bansal, A.; Agarwal, D.; Preoteasa, I. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci. Rep. 2021, 11, 3971.
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008, 58, 26–35.
- Losina, E.; Weinstein, A.M.; Reichmann, W.M.; Burbine, S.A.; Solomon, D.H.; Daigle, M.E.; Rome, B.N.; Chen, S.P.; Hunter, D.J.; Suter, E.G.; et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res. 2013, 65, 703–711.
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019, 27, 1578–1589.
- Bruyère, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.M.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.P.; et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2019, 49, 337–350.
- Machado, G.C.; Maher, C.G.; Ferreira, P.H.; Pinheiro, M.B.; Lin, C.W.; Day, R.O.; McLachlan, A.J.; Ferreira, M.L. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: Systematic review and meta-analysis of randomised placebo controlled trials. BMJ 2015, 350, h1225.
- Fransen., M.; McConnell, S.; Harmer, A.R.; van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2015, 1, CD004376.
- Nelson, A.E.; Allen, K.D.; Golightly, Y.M.; Goode, A.P.; Jordan, J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin. Arthritis Rheum. 2014, 43, 701–712.
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A randomized clinical trial. JAMA 2017, 317, 1967–1975.
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759.
- Demange, M.K.; Sisto, M.; Rodeo, S. Future trends for unicompartmental arthritis of the knee: Injectables & stem cells. Clin. Sports Med. 2014, 33, 161–174.
- Zhu, Y.; Yuan, M.; Meng, H.Y.; Wang, A.Y.; Guo, Q.Y.; Wang, Y.; Peng, J. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthr. Cartil. 2013, 21, 1627–1637.
- Bennell, K.L.; Hunter, D.J.; Paterson, K.L. Platelet-Rich Plasma for the Management of Hip and Knee Osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 24.
- Shen, L.; Yuan, T.; Chen, S.; Xie, X.; Zhang, C. The temporal effect of platelet rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017, 12, 16.
- Knop, E.; Paula, L.E.; Fuller, R. Platelet-rich plasma for osteoarthritis treatment. Rev. Bras. Reumatol. Engl. Ed. 2016, 56, 152–164.
- Zhang, H.F.; Wang, C.G.; Li, H.; Huang, Y.T.; Li, Z.J. Intra-articular platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. Drug Des. Devel. Ther. 2018, 12, 445–453.
- Patel, S.; Dhillon, M.S.; Aggarwal, S.; Marwaha, N.; Jain, A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: A prospective, double-blind, randomized trial. Am. J. Sports Med. 2013, 41, 356–364.
- Smith, P.A. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am. J. Sports Med. 2016, 44, 884–891.
- Görmeli, G.; Görmeli, C.A.; Ataoglu, B.; Çolak, C.; Aslantürk, O.; Ertem, K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: A randomized, doubleblind, placebo-controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 958–965.
- Lin, K.Y.; Yang, C.C.; Hsu, C.J.; Yeh, M.L.; Renn, J.H. Intra-articular injection of platelet-rich plasma is superior to hyaluronic acid or saline solution in the treatment of mild to moderate knee osteoarthritis: A randomized, double-blind, triple-parallel placebo-controlled clinical trial. Arthreoscopy 2019, 35, 106–117.
- Elik, H.; Doğu, B.; Yılmaz, F.; Begoğlu, F.A.; Kuran, B. The efficiency of platelet rich plasma treatment in patients with knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2020, 33, 127–138.
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715.
- Sundman, E.A.; Cole, B.J.; Karas, V.; della Valle, C.; Tetreault, M.W.; Mohammed, H.O.; Fortier, L.A. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am. J. Sports Med. 2014, 42, 35–41.
- Sakata, R.; McNary, S.M.; Miyatake, K.; Lee, C.A.; van den Bogaerde, J.M.; Marder, R.A.; Reddi, A.H. Stimulation of the superficial zone protein and lubrication in the articular cartilage by human platelet-rich plasma. Am. J. Sports Med. 2015, 43, 1467–1473.
- Khatab, S.; van Buul, G.M.; Kops, N.; Bastiaansen-Jenniskens, Y.M.; Bos, P.K.; Verhaar, J.A.; van Osch, G.J. Intra-articular injections of platelet-rich plasma releasate reduce pain and synovial inflammation in a mouse model of osteoarthritis. Am. J. Sports Med. 2018, 46, 977–986.
- De Santis, M.; di Matteo, B.; Chisari, E.; Cincinelli, G.; Angele, P.; Lattermann, C.; Filardo, G.; Vitale, N.D.; Selmi, C.; Kon, E. The role of Wnt pathway in the pathogenesis of OA and its potential therapeutic implications in the field of regenerative medicine. Biomed. Res. Int. 2018, 2018, 1–8.
- Liu, X.; Wang, L.; Ma, C.; Wang, G.; Zhang, Y.; Sun, S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/beta-catenin signaling pathway. J. Orthop. Surg. Res. 2019, 14, 470.
- Yang, J.; Guo, A.; Li, Q.; Wu, J. Platelet-rich plasma attenuates interleukin-1beta-induced apoptosis and inflammation in chondrocytes through targeting hypoxia-inducible factor-2alpha. Tissue Cell 2021, 73, 101646.
- Sun, X.; Mi, L.; Du, G.; Sun, C.; He, S. Platelet-rich plasma treatment alleviates osteoarthritis-related pain, inflammation, and apoptosis by upregulating the expression levels of microRNA-375 and microRNA-337. Immunopharmacol. Immunotoxicol. 2021, 30, 1–12, (Online ahead of print).
- Sheean, A.J.; Anz, A.W.; Bradley, J.P. Platelet-rich plasma: Fundamentals and clinical applications. Arthroscopy 2021, 37, 2732–2734.
- Uchiyama, R.; Toyoda, E.; Maehara, M.; Wasai, S.; Omura, H.; Watanabe, M.; Sato, M. Effect of platelet-rich plasma on M1/M2 macrophage polarization. Int. J. Mol. Sci. 2021, 22, 2336.
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The effect of platelet-rich plasma on the intra-articular microenvironment in knee osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5492.
- Tucker, J.D.; Goetz, L.L.; Duncan, M.B.; Gilman, J.B.; Elmore, L.W.; Sell, S.A.; McClure, M.J.; Quagliano, P.V.; Martin, C.C. Randomized, placebo-controlled analysis of the knee synovial environment following platelet-rich plasma treatment for knee osteoarthritis. PM&R 2021, 13, 707–719.
- Yurtbay, A.; Say, F.; Çinka, H.; Ersoy, A. Multiple platelet-rich plasma injections are superior to single PRP injections or saline in osteoarthritis of the knee: The 2-year results of a randomized, double-blind, placebo-controlled clinical trial. Arch. Orthop. Trauma Surg. 2021. (Online ahead of print).
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: The RESTORE randomized clinical trial. JAMA 2021, 326, 2021–2030.
- Dório, M.; Pereira, R.M.R.; Luz, A.G.B.; Deveza, L.A.; de Oliveira, R.M.; Fuller, R. Efficacy of platelet-rich plasma and plasma for symptomatic treatment of knee osteoarthritis: A double-blinded placebo-controlled randomized clinical trial. BMC Musculoskelet. Disord. 2021, 22, 822.
- Kim, J.H.; Park, Y.B.; Ha, C.W.; Roh, Y.J.; Park, J.G. Adverse reactions and clinical outcomes for leukocyte-poor versus leukocyte-rich platelet-rich plasma in knee osteoarthritis: A systematic review and meta-analysis. Orthop. J. Sports Med. 2021, 9, 23259671211011948.
- Nie, L.Y.; Zhao, K.; Ruan, J.; Xue, J. Effectiveness of platelet-rich plasma in the treatment of knee osteoarthritis: A meta-analysis of randomized controlled clinical trials. Orthop. J. Sports Med. 2021, 9, 2325967120973284.
- Li, W.; Pan, J.; Lu, Z.; Zhu, H.; Guo, J.; Xie, D. The application of platelet-rich plasma in the treatment of knee osteoarthritis: A literature review. J. Orthop. Sci. 2021, 0949, in press.
- Hong, M.; Cheng, C.; Sun, X.; Yan, Y.; Zhang, Q.; Wang, W.; Guo, W. Efficacy and safety of intra-articular platelet-rich plasma in osteoarthritis knee: A systematic review and meta-analysis. Biomed. Res. Int. 2021, 2021, 2191926.
- Aiyer, R.; Noori, S.; Schirripa, F.; Schirripa, M.; Aboud, T.; Jain, S.; Gulati, A.; Puttanniah, V.; Gungor, S.; Hunter, C. Treatment of knee osteoarthritic pain with platelet-rich plasma: A systematic review of clinical studies. Pain Manag. 2021, 11, 419–431.
- Moton, R.Z.; Nawaz, M.Z.; Latif, M.; Akhund, M.A.; Khan, Z. Clinical and functional outcomes following platelet rich plasma in the management of knee osteoarthritis: A case series in a tertiary care hospital. J. Pak. Med. Assoc. 2021, 71, S94–S98.
- Sun, S.F.; Hsu, C.W.; Lin, H.S.; Liou, I.H.; Chou, Y.C.; Wu, S.Y.; Huang, H.Y. A single intraarticular platelet-rich plasma improves pain and function for patients with early knee osteoarthritis: Analyses by radiographic severity and age. J. Back Musculoskelet. Rehabil. 2021. (Online ahead of print).
- Bec, C.; Rousset, A.; Brandin, T.; François, P.; Rabarimeriarijaona, S.; Dumoulin, C.; Heleu, G.; Grimaud, F.; Veran, J.; Magalon, G.; et al. A retrospective analysis of characteristic features of responders and impaired patients to a single injection of pure platelet-rich plasma in knee osteoarthritis. J. Clin. Med. 2021, 10, 1748.
- Hegaze, A.H.; Hamdi, A.S.; Alqrache, A.; Hegazy, M. Efficacy of platelet-rich plasma on pain and function in the treatment of knee osteoarthritis: A prospective cohort study. Cureus 2021, 13, e13909.
- Rai, D.; Singh, J.; Somashekharappa, T.; Singh, A. Platelet-rich plasma as an effective biological therapy in early-stage knee osteoarthritis: One year follow up. SICOT J. 2021, 7, 6.
- Jayaram, P.; Kang, G.E.; Heldt, B.L.; Sokunbi, O.; Song, B.; Yeh, P.C.; Epstein, M.; Shybut, T.B.; Lee, B.H.; Najafi, B. Novel assessment of leukocyte-rich platelet-rich plasma on functional and patient-reported outcomes in knee osteoarthritis: A pilot study. Regen. Med. 2021, 16, 823–832.
