Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Environmental Sciences
Plant growth-promoting bacteria (PGPR) are soil bacteria living in the rhizosphere which, through the secretion of various regulatory molecules, are involved in promoting plant growth and development. They can be found associated with the roots (rhizosphere), with the leaves (phyllosphere), or within the plant (endosphere). The endophytes (PGPE) are generally the most effective in supporting growth; being inside the plant tissues, they can communicate with the host plant and exert their beneficial effect much more efficiently.
- phytoremediation
- rhizosphere
- plant-microbe interaction
- metal uptake
- hydrocarbon rhizodegradation
- marginal soils
- drought
- salinity
1. Properties and Potential of Plant Growth-Promoting Rhizobacteria (PGPR)
Plant growth-promoting bacteria (PGPR) are soil bacteria living in the rhizosphere which, through the secretion of various regulatory molecules, are involved in promoting plant growth and development. They can be found associated with the roots (rhizosphere), with the leaves (phyllosphere), or within the plant (endosphere). The endophytes (PGPE) are generally the most effective in supporting growth; being inside the plant tissues, they can communicate with the host plant and exert their beneficial effect much more efficiently [1]. Furthermore, the PGPE, protected from the external environment, are much less subject to the soil’s frequent chemical-physical biotic and abiotic variations. Endophytic bacteria come from the rhizosphere ecosystem surrounding the roots and penetrate into plant tissues mainly by using natural fissures created in the roots during growth. The exudates and radical metabolites produced by plants represent an essential resource capable of selecting and attracting the most beneficial bacteria [2]. The profitable action of the bacteria associated with plants is mainly expressed by directly promoting the absorption of nutrients through the modulation of the levels of plant hormones. The most important and studied direct mechanisms are nitrogen fixation [3], the solubilization of inorganic phosphate [4], auxins (in particular 3-indole acetic acid, IAA [5]), cytokinins, and gibberellins [6]; the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase [7] and the production of siderophore molecules [8] (Figure 1). Instead, indirect mechanisms are defined as the inhibitory activities when the growth-promoting bacteria hinder the negative effect of pathogenic organisms (biotic stress). Some of the most common indirect mechanisms are the production of hydrogen cyanide, antibiotics, and enzymes capable of attacking and degrading the cell wall of pathogens [9].
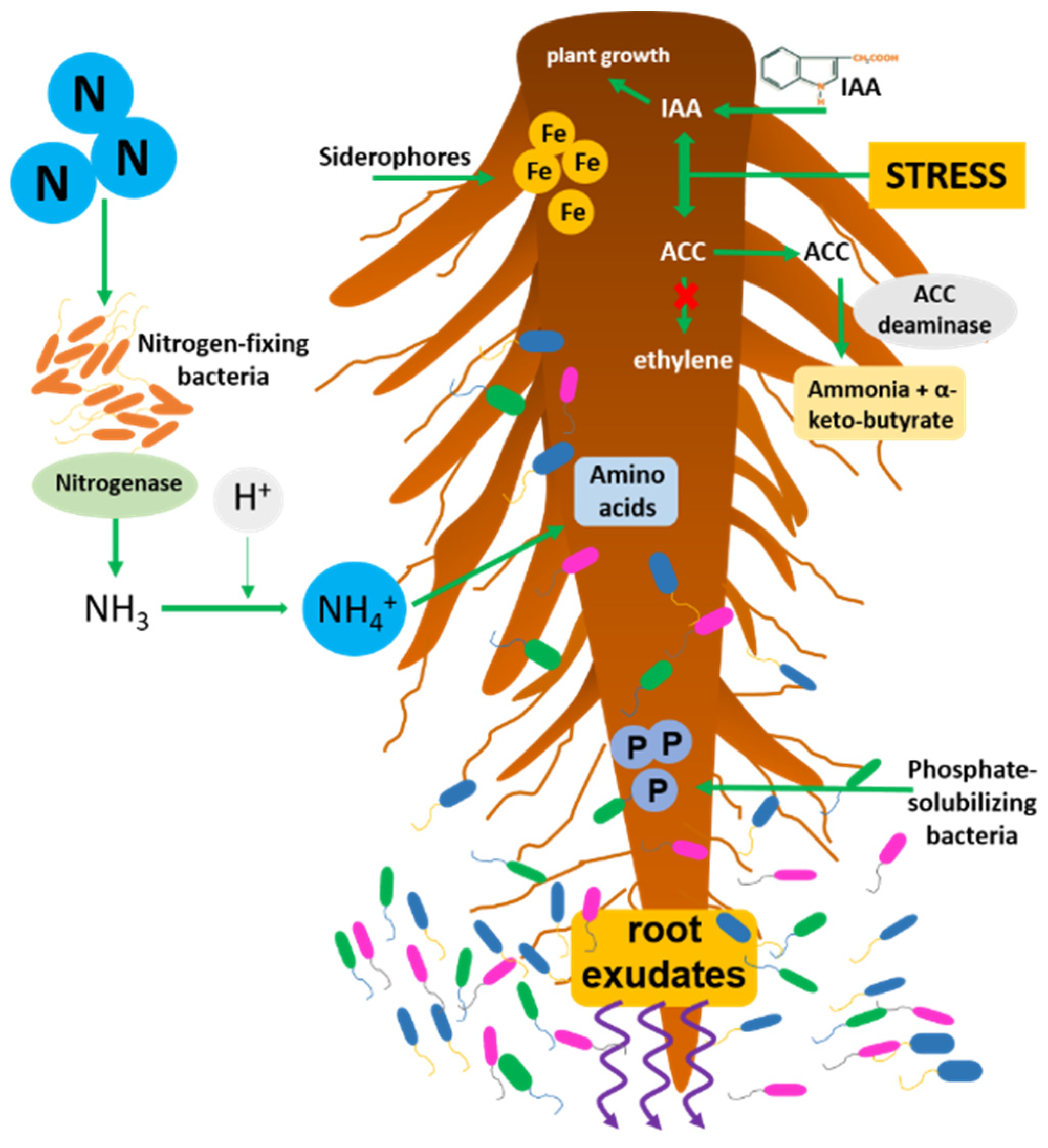
Figure 1. Simplified scheme of the main activities of PGPR and their interactions with the root system; nitrogen-fixation, phosphate solubilization, iron uptake by siderophores, ACC deaminase activity lowering ethylene levels, IAA production stimulating plant cell growth.
1.1. Siderophores
Since iron (Fe) is often present in the soil as a trivalent Fe3+ insoluble hydroxide, plants do not easily assimilate it. Small siderophore molecules are produced and secreted by bacteria (and fungi and plants) that facilitate iron absorption in the plant cell. From the chemical point of view, there are four chemical types of siderophores (catecholates, phenolates, hydroxamates, and carboxylates), but mixtures of the various types are also very common [10]. Siderophores produced by PGPR have a very high affinity for iron and can therefore sequester even small amounts of that element [11]. Bacterial siderophores can also increase metals bioavailability, favoring their uptake by plants through phytoextraction [12][13].
1.2. Phosphate Solubilization
Phosphorus (P) is an essential element for plant growth but is scarcely available in the soil. P occurs naturally in organic (Po, average 50%) and inorganic (Pi, average 50%) forms [14]. They are both not very soluble (generally no more than 5%) and therefore cannot be absorbed by the roots. The action that soil bacteria can carry out is fundamental for the solubilization and mineralization of P [15]. Plants can absorb monobasic (H2PO−4) and dibasic (HPO−24) ions. The solubilization of Pi occurs mainly with the decrease of pH in the soil due to the production of low molecular weight organic acids. The mineralization of organic phosphorus, on the other hand, occurs through the hydrolysis of phosphoric esters by phosphatase [4].
The availability of sufficient quantities of P following the activity of phosphate solubilizing bacteria (PSB) could lead to a significant decrease in the use of chemical fertilizers. The beneficial effect on plants also promotes greater efficiency of phytoextraction or phytostabilization in the presence of heavy metals.
1.3. Nitrogen Fixation
Nitrogen (N) is also an essential element for plant growth. Although atmospheric nitrogen is present in high quantities (78%), it is not found in the form available for absorption by the roots from the soil. This is mainly due to the biological nitrogen fixation (BNF) mediated by an enzymatic reaction catalyzed by the enzyme nitrogenase, transforming the atmospheric N2 into ammonia (NH3). BNF can be exerted by bacteria living in symbiotic association with legumes or higher plants, such as alder (Alnus spp.), feeding the associated nitrogen-fixing bacteria, or it can also occur in a non-symbiotic relationship through heterotrophic or autotrophic bacteria free-living in the rhizosphere [16][17]. Among the free-living (non-symbiotic) bacteria, we remember the cyanobacteria (or blue-green algae), Anabaena and Nostoc, and genera, such as Azotobacter, Beijerinckia, and Clostridium. Among the symbiotic bacteria, there are the genera Rhizobium, associated with leguminous plants; Frankia, associated with some dicotyledonous species; and some Azospirillum, associated with grasses [3][18]. In Leguminosae, colonization and subsequent invasion of rhizobia leading to active nodules containing symbiotic nitrogen-fixing bacteria can occur through root hairs or wounds in root tissue or even through intact plant cells [19].
1.4. Auxins, Cytokinins, Gibberellins
The phytohormone IAA is the most common auxin among plant-associated bacteria and play a central role in plant–bacterial interactions [20]. The main beneficial effect is root augmentation, especially root hairs and secondary roots, which consequently leads to an increase in root exudates; therefore, the production of IAA is certainly one of the most important mechanisms that favor plant growth [21]. The greater the root surface, the better the absorption of minerals from the soil. It also increases the valuable surface for the colonization of bacteria attracted by plant metabolites.
Cytokinins play a central role in developing the vascular system, in embryogenesis, in the formation of nodules, and in response to environmental changes [22]. Gibberellins are involved in the transport of metabolites in the formation of chloroplasts, leaf senescence, cell division, and stem morphogenesis [23]. While the biosynthetic pathways of gibberellins in plants and fungi have been thoroughly investigated and elucidated, little has been discovered about the biosynthesis of these enzymes in bacteria. Recent studies [24] hypothesize that bacteria have developed an independent biosynthetic pathway for the production of gibberellins.
1.5. ACC Deaminase
The occurrence of the ACC deaminase enzyme is another important direct mechanism of plant growth promotion [25]. The action of this enzyme is carried out with the inactivation of the ethylene precursor ACC, generating ammonia and alpha-ketobutyrate. High levels of ethylene can inhibit plant growth and even kill them. During saline or other environmental stress, ACC synthase and ACC oxidase activity increase ethylene synthesized by the plant. Therefore, the bacterial enzyme ACC deaminase helps plants decrease the negative effects of stress by facilitating adaptation and survival [26].
It is also interesting to underline the interaction between the activity of the ACC deaminase enzyme and the action of auxin IAA because the latter can activate the transcription of the ACC synthase enzyme, which leads to an increase in ethylene levels. The increase in the level of ethylene leads to an inhibitory response to the production of IAA, which inhibits the positive effect on plant growth. However, the presence of the ACC deaminase enzyme, which lowers the level of ethylene, counteracts the inhibition, as mentioned earlier. Many of the PGPB recognized to positively affect metals contamination in constructed wetlands can synthesize both ACC deaminase and IAA [27].
1.6. Indirect Mechanisms
The mechanisms that indirectly promote plant growth counteract pathogens’ detrimental effects [28]. Bacteria that perform this can produce antibiotics [29] or lytic enzymes degrading the cell wall. In addition to making these harmful substances for phytopathogens, PGPR can act by contending with them for the same nutrients and root colonization sites [30][31], reducing the proliferation of pathogens or even producing small amounts of hydrogen cyanide (HCN). HCN often collaborates with other biocontrol mechanisms that PGPR implement [9].
2. Effectiveness of PGPR in Hydrocarbons and Heavy Metals Contaminated Soils
PGPR can have wildly different levels of effectiveness in aiding remediation depending on many of the biological and chemical properties of the contaminated soil, ranging from presence and ratio of nutrients (e.g., N and P, competition and predation, mutation, horizontal genes transfer), to metal ions availability, quantity, and type of contaminant, and moisture. High oxygen concentration can generally help decompose organic pollutants since most degrading pathways oxidase these chemicals; it is, however, mandatory to remember that many microorganisms cannot tolerate even low oxygen levels. The addition of this nutrient could potentially kill beneficial organisms. It is also hard to overestimate the effect of temperature and pH in determining an organism survival and reproductive rates, enzymes activity, and metabolic processes, as well as the bioavailability of contaminants and their chemical form [32]. For this reason, many sites polluted by hydrocarbon are added with nutrients (nitrogen, phosphorus, potassium, etc.) and high-energy electron acceptors (especially oxygen). Some of these chemicals can be provided by plants through roots exudates. This sets up a positive feedback loop, where plants allow the presence and growth of PGPR and bacteria render the soil less toxic and aid plant growth through several mechanisms [33].
It is important to remember that petroleum hydrocarbons (PHC) are not degraded with the same efficiency. The microorganisms begin to degrade the simpler hydrocarbons (n-alkanes) first, followed by the branched ones, cyclic alkanes, to finally arrive at the most complex hydrocarbons (polyaromatics hydrocarbons PAH) [33][34]. This means that molecules, such as naphthalene, phenanthrene, and pyrene are not easy to get rid of through biodegradation.
For such molecules, the presence of plants (or phytoremediation) might provide a significant difference in degradation rates. For example, in a study conducted to observe the phytoremediation capability of Trigonella foenum-graceum and Brassica juncea in the presence of PGPR on saline soil contaminated with 400 mg/kg of phenanthrene, it was possible to reach a 99% of dissipation rate in 60 days [35].
However, many organic compounds can be highly toxic for plants, so much so that it could undermine the success of the phytoremediation. Bacteria can significantly decrease the harmful effect of many such compounds. They achieve this effect through nitrogen fixation and mobilization of nutrients, prevention of ethylene production (ACC deaminase activity), and the direct production of phytohormones. This is why it is important to create interaction between PGPR and the host plant [36] since they can suppress the inhibition of germination caused by contaminants [37]. It is, however, important to distinguish between two types of root-associated bacteria: those who remain close to or on the root surface are referred to as rhizospheric bacteria, while those that manage to enter the root tissue are called endophytes. Since the latter are protected by the root, they can influence the host plant more directly while living in a homeostatic environment, generally providing better support for degrading contaminants [38][39].
Organic compounds are not the only toxic pollutants for plants; heavy metals can cause massive changes in soil’s chemical-physical proprieties.
Plants need to produce a homeostatic network to control absorption, accumulation, and the oxidative stress that derives from heavy metals presence [40]; to do this, plants trigger many physiological and molecular mechanisms, such as active transport of ions into cell vacuoles, sequestration of metal-siderophore complexes, and production of root exudates to solubilize mineral nutrient and stimulate microbial growth [12][41].
Although metals are often present as insoluble salt and need to be mobilized by chemicals, such as EDTA (Ethylenediaminetetraacetic acid) and EDDS (Ethylenediamine-N,N-disuccinic acid), PGPR can enhance phytoextraction by reducing the toxicity of heavy metals in plants through helping control absorption, possible accumulation, or detoxification of heavy metals [41].
Those microorganisms can tolerate a high concentration of metals and can modify metal bioavailability by releasing chelants, such as organic acids and siderophores, and changing the soil pH [12]. Several studies report the isolation of microbial strains that effectively support phytoextraction or phytostabilization of various heavy metals. Among these, for example, Bacillus pumilus [12], Rhodococcus erythropolis [42], Bradyrhizobium sp. [43], Ralstonia eutropha, and Chryseobacterium humi [44].
Some PGPR, such as Sinorhizobium meliloti (a metal resistant rhizobium), can also, while co-inoculated with other PGPR, reduce the oxidative stress that some metals, such as Cu, cause to plants; this can greatly increase the metal intake during phytoextraction [45]. This effect may be attributed to the ability of rhizobium and PGPR to provide balanced nutrition to the host plant [46].
The inoculated plants had considerably lower ROS (reactive oxygen species) accumulation and a higher level of antioxidants enzymes, specifically peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX). Other studies seem to align with this result even when considering multiple growth parameters: plant biomass, the fluorescence of photosynthetic pigments, number of leaves, and shoot and root length [47][48].
However, some studies suggest that by combining the effect of both PGPR and a chelating agent, it is possible to achieve the best phytoextraction result [49][50][51][52]. In the first study, the Cu concentration in the shoots was up to 4.2 times higher in plants that were both inoculated with PGPR and treated with EDTA compared to the control. It is important to note that EDTA alone was unable to increase the biomass, likely due to the increased bioavailability of the contaminant. This effect is observable even at a high contaminant concentration [53].
This entry is adapted from the peer-reviewed paper 10.3390/app12031231
References
- Giauque, H.; Connor, E.W.; Hawkes, C.V. Endophyte traits relevant to stress tolerance, resource use and habitat of origin predict effects on host plants. New Phytol. 2019, 221, 2239–2249.
- Sørensen, J.; Sessitsch, A. Plant-associated bacteria lifestyle and molecular interactions. In Modern Soil Microbiology, 2nd ed.; van Elsas, J.D., Trevors, J.T., Jansson, J.K., Nannipieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 211–236.
- Pankievicz, V.C.S.; Irving, T.B.; Maia, L.G.S.; Ané, J. Are we there yet? The long walk towards the development of efficient symbiotic associations between nitrogen-fixing bacteria and non-leguminous crops. BMC Biol. 2019, 17, 99.
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971.
- Duca, D.R.; Glick, B.R. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619.
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the crossroads of abiotic stress signalling Pathways. Int. J. Mol. Sci. 2018, 19, 2450.
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria with Multifarious plant growth promoting traits alleviates salinity stress in french bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506.
- Radzki, W.; Gutierrez Mañero, F.J.; Algar, E.; Lucas García, J.A.; García-Villaraco, A.; Ramos Solano, B. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie Van Leeuwenhoek 2013, 104, 321–330.
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197.
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657.
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. Int. 2016, 23, 3984–3999.
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25.
- Guo, J.; Muhammad, H.; Lv, X.; Wei, T.; Ren, X.; Jia, H.; Atif, S.; Hua, L. Prospects and applications of plant growth promoting rhizobacteria to mitigate soil metal contamination: A review. Chemosphere 2020, 246, 125823.
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005.
- Liang, J.; Klingl, A.; Lin, Y.Y.; Boul, E.; Thomas-Oates, J.; Marín, M. A subcompatible rhizobium strain reveals infection duality in Lotus. J. Exp. Bot. 2019, 70, 1903–1913.
- Sickerman, N.S.; Hu, Y.; Ribbe, M.W. Nitrogenases. Methods Mol. Biol. 2019, 1876, 3–24.
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379.
- Singh, R.K.; Singh, P.; Li, H.B.; Yang, L.T.; Li, Y.R. Soil–Plant–Microbe Interactions: Use of Nitrogen-Fixing Bacteria for Plant Growth and Development in Sugarcane. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D., Singh, H., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 35–59.
- Brewin, N.J. Plant cell wall remodelling in the rhizobium–legume symbiosis. Crit. Rev. Plant Sci. 2004, 23, 293–316.
- Luo, J.; Zhou, J.J.; Zhang, J.Z. Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int. J. Mol. Sci. 2018, 19, 259.
- Grobelak, A.; Napora, A.; Kacprzak, M. Using plant growth-promoting rhizobacteria (PGPR) to improve plant growth. Ecol. Eng. 2015, 84, 22–28.
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045.
- Rizza, A.; Jones, A.M. The makings of a gradient: Spatiotemporal distribution of gibberellins in plant development. Curr. Opin. Plant Biol. 2019, 47, 9–15.
- Nett, R.S.; Montanares, M.; Marcassa, A.; Lu, X.; Nagel, R.; Charles, T.C.; Hedden, P.; Rojas, M.C.; Peters, R.J. Elucidation of gibberellin biosynthesis in bacteria reveals convergent evolution. Nat. Chem. Boil. 2017, 13, 69–74.
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39.
- del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439.
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374.
- Kundan, R.; Pant, G.; Jadon, N.; Agrawal, P.K. Plant growth promoting rhizobacteria: Mechanism and current prospective. J. Fertil. Pestic. 2015, 6, 155.
- Suárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-Growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Front. Microbiol. 2019, 10, 290.
- Barahona, E.; Navazo, A.; Martínez-Granero, F.; Zea-Bonilla, T.; Pérez-Jiménez, R.M.; Martín, M.; Rivilla, R. Pseudomonas fluorescens F113 mutant with enhanced competitive colonization ability and improved biocontrol activity against fungal root pathogens. Appl. Environ. Microbiol. 2011, 77, 5412–5419.
- Tsegaye, Z.; Gizaw, B.; Tefera, G.; Feleke, A.; Chaniyalew, S.; Alemu, T.; Assefa, F. Isolation and biochemical characterization of Plant Growth Promoting (PGP) bacteria colonizing the rhizosphere of Tef crop during the seedling stage. J. Plant Sci. Phytopathol. 2019, 3, 013–027.
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Wassie, M. The role of microorganisms in bioremediation—A Review. Open J. Environ. Biol. 2017, 2, 38–46.
- Gkorezis, P.; Daghio, M.; Franzetti, A.; Van Hamme, J.D.; Sillen, W.; Vangronsveld, J. The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: An environmental perspective. Front. Microbiol. 2016, 7, 1836.
- Tyagi, M.; da Fonseca, M.M.R.; de Carvalho, C. Bioaugmentation and biostimulation strategies to improve the effectivenesss of bioremediation processes. Biodegradation 2011, 22, 231–241.
- Urana, R.; Dahiya, A.; Sharma, P.; Singh, N. Effects of plant growth promoting rhizobacteria on phytoremediation of phenanthrene contaminated sodic soil. Polycycl. Aromat. Comp. 2019, 41, 1020–1029.
- Nie, M.; Wang, Y.; Yu, J.; Xiao, M.; Jiang, J.; Fang, C.; Chen, J.; Li, B. Understanding plant-microbe interactions for phytoremediation of petroleum-polluted soil. PLoS ONE 2011, 6, e17961.
- Franchi, E.; Agazzi, G.; Rolli, E.; Borin, S.; Chiaberge, S.; Conte, A.; Filtri, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; et al. Exploiting hydrocarbon-degrader indigenous bacteria for bioremediation and phytoremediation of a multi-contaminated soil. Chem. Eng. Technol. 2016, 39, 1676–1684.
- Lumactud, R.; Shen, S.Y.; Lau, M.; Fulthorpe, R. Bacterial endophytes isolated from plants in natural oil seep soils with chronic hydrocarbon contamination. Front. Microbiol. 2016, 7, 755.
- Marchut-Mikolajczyk, O.; Drożdżyński, P.; Pietrzyk, D.; Antczak, T. Biosurfactant production and hydrocarbon degradation activity of endophytic bacteria isolated from Chelidonium majus L. Microb. Cell Fact. 2018, 17, 171.
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412.
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Front Microbiol. 2017, 8, 1706.
- Liu, W.; Wang, Q.; Wang, B.; Hou, J.; Luo, Y.; Tang, C.; Franks, A.E. Plant growth-promoting rhizobacteria enhance the growth and Cd uptake of Sedum plumbizincicola in a Cd-contaminated soil. J. Soils Sediments 2015, 15, 1191–1199.
- Guo, J.; Chi, J. Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 2014, 375, 205–214.
- Moreira, H.; Marques, A.P.; Franco, A.R.; Rangel, A.O.; Castro, P.M. Phytomanagement of Cd-contaminated soils using maize (Zea mays L.) assisted by plant growth-promoting rhizobacteria. Environ. Sci. Pollut. Res. Int. 2014, 21, 9742–9753.
- Ju, W.; Liu, L.; Fang, L.; Cui, Y.; Duan, C.; Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226.
- Korir, H.; Mungai, N.W.; Thuita, M.; Hamba, Y.; Masso, C. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front. Plant Sci. 2017, 8, 141.
- Raklami, A.; Oufdou, K.; Tahiri, A.I.; Mateos-Naranjo, E.; Navarro-Torre, S.; Rodríguez-Llorente, I.D.; Meddich, A.; Redondo-Gómez, S.; Pajuelo, E. Safe Cultivation of Medicago sativa in metal-polluted soils from semi-arid regions assisted by heat- and metallo-resistant PGPR. Microorganisms 2019, 7, 212.
- Praburaman, L.; Park, S.H.; Cho, M.; Lee, K.; Ko, J.; Han, S.; Lee, S.; Kamala-Kannan, S.; Oh, B. Significance of diazotrophic plant growth-promoting Herbaspirillum sp. GW103 on phytoextraction of Pb and Zn by Zea mays L. Environ. Sci. Pollut. Res. Int. 2017, 24, 3172–3180.
- Abbaszadeh-Dahaji, P.; Baniasad-Asgari, A.; Hamidpour, M. The effect of Cu-resistant plant growth-promoting rhizobacteria and EDTA on phytoremediation efficiency of plants in a Cu-contaminated soil. Environ. Sci. Pollut. Res. Int. 2019, 26, 31822–31833.
- Hamidpour, M.; Nemati, H.; Abbaszadeh Dahaji, P.; Reza Roosta, H. Effects of plant growth-promoting bacteria on EDTA-assisted phytostabilization of heavy metals in a contaminated calcareous soil. Environ. Geochem. Health 2020, 42, 2535–2545.
- Franchi, E.; Petruzzelli, G. Phytoremediation and the key role of PGPR. In Advances in PGPR Research; Singh, H.B., Sarma, B.K., Keswani, C., Eds.; CABI: Wallingford, UK, 2017; pp. 306–329.
- Franchi, E.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Improved arsenic phytoextraction by combined use of mobilizing chemicals and autochthonous soil bacteria. Sci. Total Environ. 2019, 655, 328–336.
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40.
This entry is offline, you can click here to edit this entry!
