Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Immune checkpoint inhibitors (ICIs) such as PD1/PD-L1 blockers are an established treatment for many solid cancers. There are currently no approved ICIs for sarcomas, but satisfactory results have been seen in some patients with disseminated disease in certain histological types. Most studies on PD-L1 in sarcoma have used small specimens and there are no clear cutoff values for scoring.
- sarcoma
- immunotherapy
- PD-L1
- chondrosarcoma
- liposarcoma
1. Introduction
The complex network of interactions present in the tumor microenvironment has in recent years risen, as the new frontier of targeted cancer therapies for a number of malignant tumors, with the programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway as the currently most widely utilized target. PD-L1 is a type 1 transmembrane protein, and a ligand to PD-1, a receptor found on T, B and myeloid cells. The binding of PD-1 and PD-L1 suppresses the adaptive immune system by interactions with phosphatases (SHP-1 or -2) via ITSM (Immunoreceptor Tyrosine-based Switch Motif [1][2]. Tumor cells utilize this mechanism to escape the immune system, which in turn, by inhibiting this bond, may properly identify and attack tumor cells. PD-L1 immunoreactivity is now part of routine workup in many solid cancers, but is not yet established in sarcomas.
Targeted treatments using immune checkpoint inhibitors (ICI) blocking the binding between PD-L1 and PD1 is already established in many solid cancers such as melanoma and lung cancer, with favorable response even in inoperable or disseminated disease [3][4]. Treatment response is often correlated with the presence of tumor-infiltrating lymphocytes (TILs) and PD-L1 expression in both tumor and immune cells [1][5]. Association with worse clinical outcome has been demonstrated in solid cancers [6][7][8] as well as soft tissue sarcomas and osteosarcoma [9][10][11]. Other studies have found PD-L1 expression to be a positive predictive marker [11], or that it had no significant impact on prognosis [12][13][14][15].
The diversity and rarity of sarcomas pose challenges; sample sizes are often small and difficult to extrapolate across subtypes. Current studies show significant variation in both PD-L1 immunoreactivity and blockade efficacy among histological subtypes, which seem to be the most important factors in that regard [16]. Sarcomas found to have PD-L1 immunoreactivity include, among others, angiosarcoma, chondrosarcoma, osteosarcoma, Ewing’s sarcoma, liposarcoma, rhabdomyosarcoma, synovial sarcoma [7], undifferentiated pleomorphic sarcoma and MPNST [16][17][18][19][20]. Currently, there are no ICIs formally approved for treatment of sarcomas, although it can be considered for specific soft tissue sarcoma subtypes based on results from phase II studies [21].
Though cutoff values have been defined in some cancers such as non-small-cell lung cancer, the predictive and prognostic values of PD-L1 expression have not yet reached a consensus in many other cancer types. There are clear protocols for scoring PD-L1 in cancers with formal indication for ICI with cutoff values depending on histological type as well as antibody assay. The lack of established protocols for evaluating PD-L1 immunohistochemistry staining in sarcomas is a major challenge, given that different histological subtypes might have different cutoff values. Existing studies often adapt pre-existing protocols [22][23], but the absence of consensus results in interstudy variation in cutoffs for positive expression.
2. Clinical Data
A total of 230 tumors from 214 patients were included: 76 tumors from 74 patients with chondrosarcoma, 58 tumors from 44 patients with liposarcoma and 96 tumors from 96 patients with undifferentiated pleomorphic sarcoma. Of the 96 patients with UPS, 18 cases were included based on ad hoc staining of PD-L1 (SP263) in the clinical setting and were not stained with SP142. The average age was 58 (range 16–83) years for CS, 69 (26–86) for LS and 70 (46–93) for UPS. Median overall survival was 11 (0,3–39) years for CS, 4 (0,1–32) for LS and 3 (0,1–12) for UPS. None of the patients had received systemic therapy with ICIs or other immune-modulating agents.
3. Chondrosarcoma
Clinical characteristics and visualization of PD-L1 staining is demonstrated in Figure 1. The majority of CS were PD-L1-negative (Table 1). PD-L1 immunoreactivity was more common in tumors with higher histological grade (grade 3 or dedifferentiated) and not observed in any grade 2 tumors. Three tumors also showed >50% immunoreactivity in TC using SP263 (Figure A1). The presence of immune cells (IC) was rare; 22 cases had IC present, and only two cases had ≥1% immunoreactivity (Table 1, Figure A1).
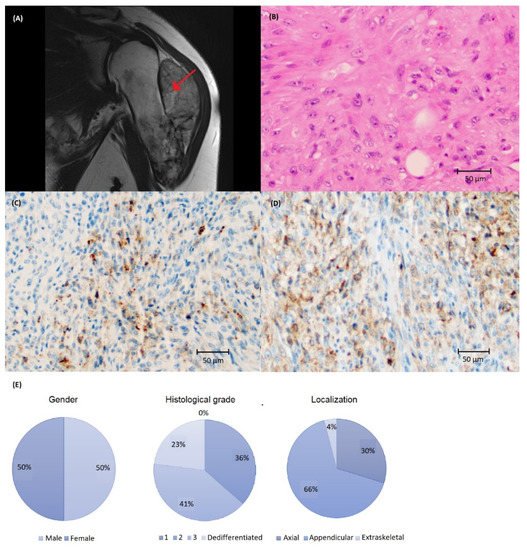
Figure 1. (A) Magnetic resonance imaging depicting chondrosarcoma (CS) in the humerus (arrow) and (B) histology of high-grade CS stained with (C) SP142 and (D) SP263 assays at ×200 magnification showing a higher number of cells with PD-L1 immunoreactivity using the SP263 assay. (E) Clinical and tumor characteristics of the CS cohort.
Table 1. PD-L1 immunoreactivity in chondrosarcomas, liposarcomas and undifferentiated pleomorphic sarcomas stained with SP142 and SP263 immunoassays (Roche).
| Sarcoma type | PD-L1 Immunoreactivity | TC (No. of Cases) |
IC (No. of Cases) |
||
|---|---|---|---|---|---|
| SP142 | SP263 | SP142 | SP263 | ||
| Chondrosarcoma | |||||
| 0 | 65 | 66 | 20 | 20 | |
| >1% | 11 | 10 | 2 | 2 | |
| Liposarcoma | |||||
| 0 | 31 | 30 | 13 | 12 | |
| <5% | 22 | 15 | 15 | 13 | |
| 5–9% | 4 | 9 | 13 | 8 | |
| ≥10% | 1 | 4 | 6 | 14 | |
| Undifferentiated pleomorphic sarcoma | |||||
| 0 | 18 | 19 | 16 | 16 | |
| <5% | 34 | 27 | 17 | 15 | |
| 5–9% | 13 | 23 | 13 | 13 | |
| ≥10% | 12 | 27 | 12 | 24 | |
Univariate analysis showed that higher age and tumor grade were significantly associated with shorter MFS and OS (Figure 2 and Figure A2). There was no significant correlation between PD-L1 immunoreactivity and clinical outcome; Kaplan–Meier analysis showed that patients with PD-L1-positive tumors had shorter OS, but the difference was nonsignificant and most likely explained by the association to high tumor grade (Figure 3).
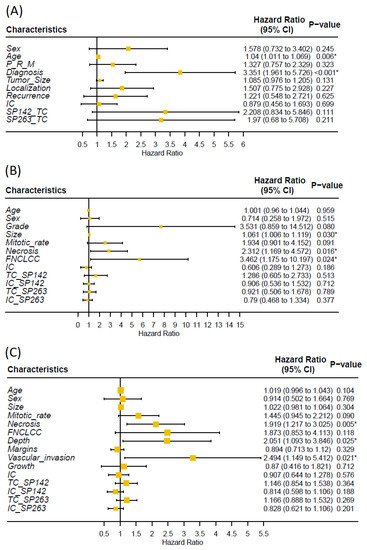
Figure 2. Univariate cox regression analysis of association between clinical/tumor characteristics, PD-L1 immunoreactivity and overall survival of (A) chondrosarcoma, (B) liposarcoma and (C) undifferentiated pleomorphic sarcoma (P = primary, M = metastasis, R = recurrence, * = p < 0.05). Researchers found no significant association between PD-L1 immunoreactivity and overall survival.
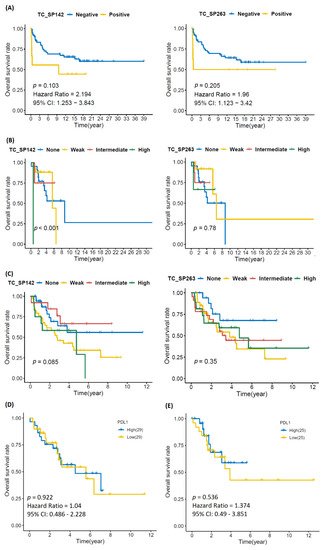
Figure 3. Kaplan–Meier curves depicting overall survival in (A) chondrosarcoma, (B) liposarcoma and (C) undifferentiated pleomorphic sarcoma in relation to PD-L1 immunoreactivity in tumor cells, as well as data from the Cancer Genome Atlas for (D) LS and (E) UPS, showing no significant impact of PD-L1 status on overall survival. Kaplan–Meier survival analysis was performed by log-rank test.
4. Liposarcoma
Clinical characteristics and visualization of PD-L1 staining is demonstrated in Figure 4. Most tumors had either none or low immunoreactivity with SP142 and none, low or intermediate with SP263 (Table 1). One tumor had >50% PD-L1 immunoreactivity in TC using SP263 (Figure A1). Immune infiltrate was present in 48 cases; 35 cases expressed ≥1% immunoreactivity with SP263 and 34 cases with SP142 (Table 1, Figure A1).
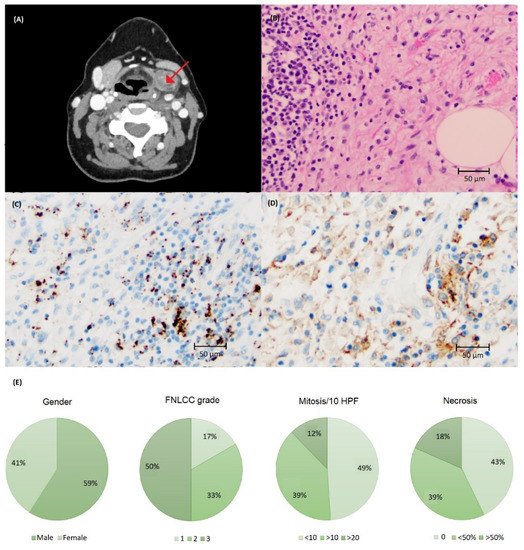
Figure 4. (A) Computed tomography of liposarcoma (LS) in the neck region (arrow). (B) Histology of LS stained with (C) SP142 and (D) SP263 assays at ×200 magnification showing a higher number of cells with PD-L1 immunoreactivity using the SP263 assay. (E) Clinical and tumor characteristics of the LS cohort.
Univariate analysis showed that the presence of necrosis and higher FNLCC grade were significant associated with shorter MFS and OS (Figure 2 and Figure A2). Kaplan–Meier showed a statistically significant reduction in MFS and OS in patients with PD-L1 immunoreactivity for the SP142 assay, but not for SP263; however, no statistically significant impact was seen using univariate analysis (Figure 2, Figure 3 and Figure A3). PD-L1 immunoreactivity in IC had no significant impact on MFS and OS (Figure A4 and Figure A5).
5. Undifferentiated Pleomorphic Sarcoma
Clinical characteristics and visualization of PD-L1 staining are demonstrated in Figure 5. PD-L1 immunoreactivity was more common in UPS (Table 1) compared to CS and LS (p < 0.05). Furthermore, >50% immunoreactivity was seen in two and eight tumors using the SP142 and SP263 assay, respectively (Figure A1). Immune cells were observed in 68 cases with 43 and 52 cases expressing ≥1% immunoreactivity with SP142 and SP263, respectively (Table 1, Figure A1).
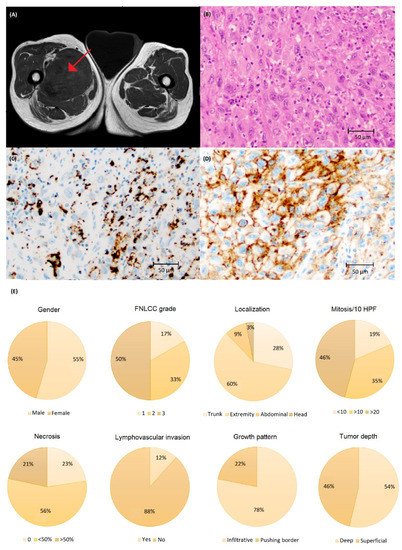
Figure 5. (A) Magnetic resonance imaging of undifferentiated pleomorphic sarcoma (UPS) in the thigh (arrow) and (B) histology of UPS stained with (C) SP142 and (D) SP263 assays at ×200 magnification showing a higher number of cells with PD-L1 immunoreactivity using the SP263 assay. (E) Clinical and tumor characteristics of the UPS cohort.
Univariate analysis showed that the presence of necrosis, tumor growth in deep tissue and vascular invasion were significantly associated with shorter MFS and OS (Figure 2 and Figure A2). Kaplan–Meier showed no significant difference in MFS and OS in patients with PD-L1-positive vs. -negative TC or IC (Figure 3, Figure A3, Figure A4 and Figure A5).
This entry is adapted from the peer-reviewed paper 10.3390/biom12020292
References
- LaFleur, M.W.; Muroyama, Y.; Drake, C.G.; Sharpe, A.H. Inhibitors of the PD-1 Pathway in Tumor Therapy. J. Immunol. 2018, 200, 375–383.
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 2019, 15, 100221.
- Mahoney, K.M.; Freeman, G.J.; McDermott, D.F. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin Ther. 2015, 37, 764–782.
- Geng, Y.; Zhang, Q.; Feng, S.; Li, C.; Wang, L.; Zhao, X.; Yang, Z.; Li, Z.; Luo, H.; Liu, R.; et al. Safety and Efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: A systematic review and meta-analysis. Cancer Med. 2021, 10, 1222–1239.
- Yi, M.; Jiao, D.; Xu, H.; Liu, Q.; Zhao, W.; Han, X.; Wu, K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer 2018, 17, 1–14.
- Ohigashi, Y.; Sho, M.; Yamada, Y.; Tsurui, Y.; Hamada, K.; Ikeda, N.; Mizuno, T.; Yoriki, R.; Kashizuka, H.; Yane, K.; et al. Clinical Significance of Programmed Death-1 Ligand-1 and Programmed Death-1 Ligand-2 Expression in Human Esophageal Cancer. Clin. Cancer Res. 2005, 11, 2947–2953.
- Massi, D.; Brusa, D.; Merelli, B.; Ciano, M.; Audrito, V.; Serra, S.; Buonincontri, R.; Baroni, G.; Nassini, R.; Minocci, D.; et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann. Oncol. 2014, 25, 2433–2442.
- Xiang, X.; Yu, P.-C.; Long, D.; Liao, X.-L.; Zhang, S.; You, X.-M.; Zhong, J.-H.; Li, L.-Q. Prognostic value of PD-L1 expression in patients with primary solid tumors. Oncotarget 2017, 9, 5058–5072.
- Zheng, C.; You, W.; Wan, P.; Jiang, X.; Chen, J.; Zheng, Y.; Li, W.; Tan, J.; Zhang, S. Clinicopathological and prognostic significance of PD-L1 expression in sarcoma: A systematic review and meta-analysis. Medicine 2018, 97, e11004.
- Bertucci, F.; Finetti, P.; Perrot, D.; Leroux, A.; Collin, F.; Le Cesne, A.; Coindre, J.-M.; Blay, J.-Y.; Birnbaum, D.; Mamessier, E. PDL1 expression is a poor-prognosis factor in soft-tissue sarcomas. OncoImmunology 2017, 6, e1278100.
- Kim, J.R.; Moon, Y.J.; Kwon, K.S.; Bae, J.S.; Wagle, S.; Kim, K.M.; Park, H.S.; Lee, H.; Moon, W.S.; Chung, M.J. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS ONE 2013, 8, e82870.
- Matikas, A.; Zerdes, I.; Lövrot, J.; Richard, F.; Sotiriou, C.; Bergh, J.; Valachis, A.; Foukakis, T. Prognostic Implications of PD-L1 Expression in Breast Cancer: Systematic Review and Meta-analysis of Immunohistochemistry and Pooled Analysis of Transcriptomic Data. Clin. Cancer Res. 2019, 25, 5717–5726.
- Toulmonde, M.; Adam, J.; Bessede, A.; Ranchère-Vince, D.; Velasco, V.; Brouste, V.; Blay, J.-Y.; Mir, O.; Italiano, A. Integrative assessment of expression and prognostic value of PDL1, IDO, and kynurenine in 371 primary soft tissue sarcomas with genomic complexity. J. Clin. Oncol. 2016, 34, 11008.
- Botti, G.; Scognamiglio, G.; Marra, L.; Pizzolorusso, A.; Di Bonito, M.; De Cecio, R.; Cantile, M.; De Chiara, A. Programmed Death Ligand 1 (PD-L1) Expression in Primary Angiosarcoma. J. Cancer 2017, 8, 3166–3172.
- Kostine, M.; Cleven, A.H.; de Miranda, N.; Italiano, A.; Cleton-Jansen, A.-M.; Bovee, J. Analysis of PD-L1, T-cell infiltrate and HLA expression in chondrosarcoma indicates potential for response to immunotherapy specifically in the dedifferentiated subtype. Mod. Pathol. 2016, 29, 1028–1037.
- Zuo, W.; Zhao, L. Recent advances and application of PD-1 blockade in sarcoma. OncoTargets Ther. 2019, 12, 6887–6896.
- Keung, E.Z.; Tsai, J.W.; Ali, A.M.; Cormier, J.N.; Bishop, A.J.; Guadagnolo, B.A.; Torres, K.E.; Somaiah, N.; Hunt, K.K.; Wargo, J.A. Analysis of the immune infiltrate in undifferentiated pleomorphic sarcoma of the extremity and trunk in response to radiotherapy: Rationale for combination neoadjuvant immune checkpoint inhibition and radiotherapy. Oncoimmunology 2018, 7, e1385689.
- Park, H.K.; Kim, M.; Sung, M.; Lee, S.E.; Kim, Y.J.; Choi, Y.-L. Status of programmed death-ligand 1 expression in sarcomas. J. Transl. Med. 2018, 16, 303.
- Veenstra, R.; Kostine, M.; Cleton-Jansen, A.-M.; de Miranda, N.; Bovée, J.V. Immune checkpoint inhibitors in sarcomas: In quest of predictive biomarkers. Lab. Investig. 2017, 98, 41–50.
- Kosemehmetoglu, K.; Ozogul, E.; Babaoglu, B.; Tezel, G.G.; Gedikoglu, G. Programmed death ligand 1 (pd-l1) expression in malignant mesenchymal tumors. Turk. J. Pathol. 2017, 1.
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021, 32, 1348–1365.
- Kim, C.; Kim, E.K.; Jung, H.; Chon, H.J.; Han, J.W.; Shin, K.-H.; Hu, H.; Kim, K.S.; Choi, Y.D.; Kim, S.; et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016, 16, 1–7.
- Kelany, M.R.; Barth, T.; Salem, D.; Mosaad, M. PD-L1 expression in soft tissue sarcomas and its prognostic implication. J. Clin. Oncol. 2019, 37, e14261.
This entry is offline, you can click here to edit this entry!
