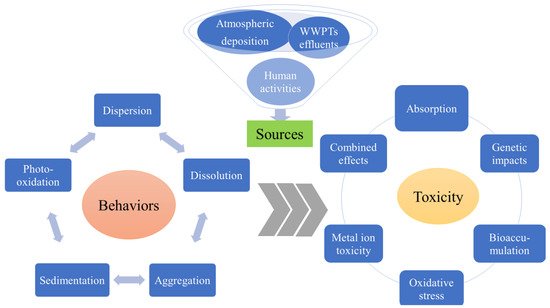
2.1. Sources and Occurrences
UVFs have been detected in surface waters [
29], urban groundwater [
30], sediments [
31,
32,
33], marine water, and biota [
1,
34]. The environmental sources and distribution of organic UVFs have been well reviewed in recent years [
1,
34]. However, very little is known about the occurrences and distributions of the two increasingly used inorganic UVFs (nTiO
2 and nZnO). It has been shown that these substances are released into waters, either directly through human activities or indirectly through wastewater treatment plant (WWTP) drainage and atmospheric deposition (shown in
Figure 1) [
11,
29,
35]. Some studies have indicated that there is a direct relationship between the amounts of sunscreen components in waters and recreational activities, such as swimming, diving, surfing, etc [
4,
36,
37]. In addition, the effluents of WWTPs and domestic sewage indirectly release UVFs, as sunscreen components cannot be completely removed [
6,
11]. Atmospheric aerosols containing UVFs may occur from different sources, including directly after spraying sunscreen on the skin, with effluents from WWTPs, and indirectly with the incineration of WWTP sludge.
Figure 1. The sources, behaviors, and toxicity of sunscreen-derived inorganic UVFs in aquatic environments.
According to a survey study, there are approximately 16,000–25,000 tons per annual (t/a) of sunscreens that contain nTiO
2 in tropical countries, and at least 25% of sunscreen applied to the skin enters the ocean during water recreational activities [
9]. It is estimated that the content of nTiO
2 in sunscreens is approximately 4%, and the amount of nTiO
2 released annually is approximately 160–250 t in these tropical countries [
1,
38]. Specifically, Sánchez-Quiles and Tovar-Sánchez [
9] estimated that over 4 kg of nTiO
2 can be released from sunscreen into seawater during a summer day on a tropical touristic beach. Another study suggested that the recreational activities that take place at Old Danube Lake (Vienna, Austria) may involve the consumption of sunscreen of 8.1 t per year, and they estimated that 94.5 kg of TiO
2 per year may be released into lake waters [
39]. A recent study has shown that inorganic UVFs present in the formulation of sunscreens are detected in nearshore water and are concentrated in the surface microlayer that ranges from 6.9 to 37.6 mg/L for TiO
2 and from 1.0 to 3.3 mg/L for ZnO [
4].
2.2. Environmental Behaviors
The specific behavior of inorganic UVFs released from sunscreens into aquatic environments has not been well addressed. As sunscreen is a complex chemical mixture; once it is in water, the inorganic UVFs released from sunscreen are complex and can exist in the form of aggregates of various complex components [
40,
41], including surface-modified complexes or raw NPs. For raw NPs, their environmental fate generally includes dispersing, aggregating, and dissolving/releasing metal ions and settling onto sediments or being absorbed and bioaccumulated by organisms (shown in
Figure 1) [
28,
39,
42]. Many studies have confirmed that nZnO UVFs rapidly dissolve in water and form hydrated Zn
2+ cations [
43,
44]. Other inorganic UVFs, e.g., nTiO
2, are regarded as relatively stable and rather insoluble in water [
45]. Thus, these UVFs tend to aggregate into larger particles, which remain suspended or precipitate to the bottom of the aquatic environment. In general, the higher the content of UVFs, the higher the SPR the sunscreen obtained. For organic UVFs, they absorb UVR, thus their spectral characteristics determined the absorbance of UVR as well as the sun protection factor (SPR); most of them are photo-instability effects related to UVR exposure [
46]. For inorganic UVFs, they mean to scatter and reflect UVR; thus, they are more stable than organic UVFs, but their particle size would affect the SPR and transparency (aesthetics of the products), thus most inorganic UVFs are nanosized. The stability of physical sunscreens was influenced by the coating materials, with these organic materials in physical sunscreens tend to perform photodegradation and photo-instability effects related to UVR exposure, thus making inorganic UVFs easier to bear in the environment [
46]. In addition, photooxidation and photodegradation are also proposed to occur when inorganic UVFs are exposed to sunlight. Inorganic UVFs, including nTiO
2 and nZnO, are often used as photocatalytic materials; once released into water, they can be photooxidized during irradiation by ultraviolet light and generate hole-electron pairs; reactive oxygen species (ROS) are produced when hole-electron pairs react with H
2O or O
2 on the surface of NPs, which also decreases the particle size and produces more ROS [
47,
48]. Studies have shown that inorganic UVFs are photooxidized, produce ROS, and cause photocatalytic toxicity to aquatic organisms [
49]. In addition to these behaviors, inorganic UVFs easily settle into sediments due to gravitational force, thereby aggregating into larger NPs. UVFs, both the organic and inorganic varieties, are absorbed or captured by aquatic organisms during the above processes, which causes damage to organisms and even bioaccumulation in organisms or sediments in the water. We recently found that physical sunscreens and related inorganic UVFs exhibit bioattachment on the surfaces of button coral and cause significant growth inhibition and expulsion of zooxanthellae (
Symbiodinium sp., unpublished data), which demonstrates the importance of further exploring the environmental fate of inorganic UVF-containing cosmetic products and the derived UVFs.
The nTiO
2 and nZnO were dispersed (partial dissolved) in physical sunscreens during the manufacturing process, which would be modified first sometimes. Thus inorganic UVFs in sunscreens often exist as surface-modified complexes. For surface-modified complexes, their potential environmental behavior presents some differences that need to be discussed. Primarily, coexisting surface coatings affect the fate of NPs to some extent. In addition to UVFs, sunscreens also contain other ingredients, such as preservatives (e.g., paraben derivates) [
50], coloring agents (e.g., ammonium sulfate, ferric ammonium ferrocyanide, copper powder, and iron) [
51], film-forming agents (e.g., acrylates and acrylamides) [
52], surfactants, chelators, viscosity controllers (e.g., potassium cetyl phosphate and pentasodium ethylenediamine tetramethylene phosphonate), and fragrances [
53]. Some of these ingredients have been detected in coastal waters [
54,
55,
56]. Thus, nTiO
2 (and nZnO) may be present in the form of bare or coated NPs in the aquatic environment, and their dimension, shape, crystal phase, and surface area vary among different sunscreen products [
27]. A recent study showed that sunscreen-derived nTiO
2 exhibits a larger particle size but a smaller hydrodynamic diameter and lower zeta potential than industrial uncoated nTiO
2, which exhibits significant aggregation [
57]. In contrast, the presence of carboxymethyl cellulose (CMC) or polyvinylpyrrolidone (PVP) significantly enhances the stability of uncoated nTiO
2, as determined by the zeta potential values measured at pH 7, with substantial shape changes that result in spherical particles and relatively small nTiO
2 sizes [
57]. Similar substantial shape transformations induced by stabilizers have been found in other studies [
58,
59]. Inorganic UVFs generally have a small particle size, strong hydrophobicity, and are insoluble in water; thus, Brownian motion, eddy motion, and runoff shear force result in some inorganic UVF particles remaining in suspension [
60]. Engineered polymers or organic and inorganic substances that serve as coating materials or act as stabilizers have been found to modify the physicochemical properties of raw NPs, thereby affecting particle stability and mobility through electrostatic repulsion [
61,
62,
63] and by maintaining the dispersion of nanosized inorganic UVFs. For example, nTiO
2 has been found to be fully dispersed and stabilized in natural water that contains organic materials [
64]. Therefore, the stability of inorganic UVFs depends on their physicochemical properties and coating materials [
27,
57].
An early study indicated that eight of nine commercial sunscreen products are coated with nonvolatile inorganic residues, typically Al
2O
3 or SiO
2, to minimize the photochemical activity of TiO
2 [
27]. Adsorbed or covalently bonded surfactants affect aggregation stability by increasing the surface charge and electrostatic repulsion or by reducing the interfacial energy between the particles and the solvent [
65]. The interaction between steric repulsion and universal Coulomb attraction is caused by the surface coating layers, which may profoundly affect the aggregation kinetics. However, a recent study showed that sodium citrate provides higher stability for spherical nTiO
2 than PVP, sodium dodecyl sulfate, and polyethylene glycol, since sodium citrate results in lower critical coagulation concentrations [
66]. Additionally, another study showed that the addition of coating materials such as CMC, PVP, and silica prevents significant TiO
2 aggregation by facilitating dispersion [
60]. These stabilizers change the physicochemical properties (particle sizes and zeta potential) of nTiO
2 and produce a stable TiO
2 suspension with a cluster size smaller than that of uncoated nTiO
2 because they play the role of a dispersant that prevents nanoparticle aggregation [
57]. A decrease in particle size results in a higher proportion of atoms on the particle surface, which alters the electronic structure, surface charge, and final degree of aggregation [
67]. Small particles with high surface energy aggregate more readily than larger particles since aggregation reduces the free energy in the NP system.
It has been revealed that the dissolution of inorganic UVFs depends on the solubility of the materials themselves and on the concentration gradient in water [
68,
69]. For example, nZnO releases more Zn ions in seawater with a higher ionic strength than in fresh water [
70]. Moreover, the dissolution of inorganic UVFs is clearly affected by the physicochemical properties of the material, such as the particle size, shape, and surface coating. Generally, the solubility of NPs is higher than that of the bulk phase because the decreased size increases the specific surface areas and the enthalpies of the formation of the ions [
71]. Fairbairn et al. [
72] also pointed out that nZnO is more easily dissolved in sea water than ZnO with ordinary particle sizes or Fe-doped nZnO. However, for nZnO, the impact of different sizes on dissolution is not as obvious for nanosized, bulk, or large particles due to the high solubility of ZnO, which can exhibit up to 80% dissolution [
69,
73,
74]. Additionally, the shapes of NPs have been shown to affect both the rates of dissolution and the equilibrium concentrations [
14]. The dissolution rate for spherical nCuO is faster than that of rod and spindle nCuO [
75], while spherical nZnO induces lower toxicity than rod-shaped nZnO because the actual Zn ion concentration that results from the dissolution of rod-shaped nZnO is much higher than that of spherical nZnO [
76].
Quite often, the dissolution rate of inorganic UVFs significantly decreases in the presence of surface coatings because the surface coating acts as a physical barrier or shield that prevents electrons or photons from reaching the NP surface [
77]. In sunscreens, photoactivity problems may arise if particles are not treated with coatings, and manufacturers commonly employ inert surface coatings that dramatically reduce the potential for photoactivity; existing data suggests that these surface coatings reduce UV reactivity by as much as 99% [
40,
41]. However, organic coatings slow the dissolution process relative to that of uncoated ZnO but lead to an increased concentration of Zn
2+ at equilibrium [
78]. Otherwise, if the coatings are not stable or if manufacturers use forms of ZnO or TiO
2 that are not optimized for stability and sun protection, then sunscreens may not be protective [
14]. These results suggest that inorganic UVFs might input substantial amounts of free metals into an aquatic environment and pose a toxicity risk to aquatic ecosystems.
In addition to the influence of internal NP properties, external environmental factors such as light, pH, and natural organic matter (NOM) can also make a difference. The interaction energy barrier decreases with a decreasing particle size according to the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory, and it is affected by the properties of the primary NPs (e.g., size, shape, chemical composition, and surface coatings), solution chemistries (e.g., pH, ionic identity, electrolyte patterns, and reactions with NOM), and environmental conditions (e.g., temperature and dissolved oxygen level) [
69,
79]. For example, a large proportion of nZnO dissolves at a limit close to the solubility limit of ZnO(s) at a high pH of approximately 8.2, and both visible and UV light facilitate nZnO dissolution at lower pH values that range from 4.8 to 6.5 [
80]. Light warms the water, enhances the release rates of inorganic UVFs, shortens the equilibrium time and even increases equilibrium concentrations [
62]. Moreover, inorganic UVFs generate ROS under irradiation with visible and UV light; this results in the oxidation of metal ions and surface organic compounds, which increases the dissolution rates due to the decomposition of surface coatings and loss of the stabilizing effect of dissolved organic matter. The influence of solution properties on the dissolution of inorganic UVFs is dynamic and complex [
62].
2.3. Substantial Environmental Impacts
The discharge of inorganic UVFs from sunscreens into waters is concomitant with the input of several other constituents, including nutrients (e.g., silicates, phosphates, and nitrates), metals (e.g., Al, Cd, Cu, Co, Mn, Mo, Ni, Pb, and Ti), and coating materials (e.g., preservatives, coloring agents, film-forming agents, surfactants, and stabilizers). Many of these coexisting substances are persistent; therefore, their effects might last beyond the most recent period of sunscreen use. These additional constituents influence the bioavailability and degradability of sunscreen ingredients since the biogeochemical routes into environmental media (water, sediment, and biota) and the hydrophobicity or hydrophilicity of the substances contained in sunscreens are diverse and complex [
1,
81]. Moreover, the effects of sunscreen contamination (especially from commercial formulations instead of individual compounds or ingredients) are sometimes difficult to perceive in laboratory studies because of their complex matrix [
82,
83] and unknown composition [
84]. Additionally, because of the diverse formats of sunscreens (e.g., cream, gel, spray, and oil), their dilution and release of UVFs into water are different, as are their bioavailabilities and toxicities [
4,
85].
It is likely that environmental exposure to inorganic UVFs and the chemicals contained therein results from the production and consumption of sunscreens. Studies have indicated that UVFs and other ingredients from sunscreens have been detected in the tissues of marine organisms, such as clams, oysters, gastropods, and fish [
86,
87], and have shown toxicity in some aquatic species, such as the crustacean
Daphnia pulex and the fish
Danio rerio [
88,
89]. Rodríguez-Romero et al. [
90] demonstrated with laboratory experiments and field measurements that sunscreens are an important source of nutrients, such as nitrogen compounds (NO
3−, NO
2−, and NH
4+) and phosphate (PO
43−) in coastal marine environments, raising the possibility of algal blooms in oligotrophic waters. More specifically, some concentrations of the compounds (e.g., those of PO
43-, NH
4+, NO
3−, and Ti) released into water vary during the course of a day, which is known to be associated with variations in beach-goer activities and changes in solar radiation [
4]. Sunscreens have also been identified as sources of high-risk metal substances [
91], many of which (e.g., Al, Zn, Mg, Fe, Mn, Cu, Cr, and Pb) have been detected and quantified in aquatic environments [
4,
92]. Moreover, the organic components of sunscreens are readily removed from particle surfaces [
93,
94], which leaves the inorganic UVFs exposed to the surrounding environment. Although the ecological relevance of this input has not been well reviewed, Tovar-Sánchez et al. [
4] suggested that it could enhance primary production in the oligotrophic waters of the Mediterranean Sea.
In addition to the direct output of soluble substances from sunscreens, some indirect metabolites are also produced in the water environment under sunlight. A study carried out on a touristic beach indicated that both temporal (daily) and vertical (water column) distributions of H
2O
2 concentrations generated by inorganic UVFs (nTiO
2 and nZnO) were present in marine waters [
9]. According to the authors, the concentrations of H
2O
2 found within the top centimeter of the surface layer were up to 41.6% higher than those in the immediate subsurface waters [
9]. Similarly, a large number of studies have indicated that nTiO
2 and nZnO produce ROS under sunlight exposure and induce oxidative stress in organisms [
62,
95,
96,
97,
98]. Therefore, more reliable information is required on the role of sunlight in the release of the main ingredients and byproducts of sunscreens into water.
Accordingly, sunscreen-derived inorganic UVFs are very likely to be released into the main water bodies of lakes, rivers, and oceans but do not remain suspended for a long time, with the most likely fates being aggregation, dissolution, and settling onto the sediments due to the water chemistry conditions and the presence of natural colloids. However, their environmental behaviors will be affected by the surface coating and various physical and chemical factors, such as ocean currents, waves, and high salinity, and they will undergo complex aggregation and dissolution reactions; moreover, their structural form, distribution, and toxic effects will constantly change. Nevertheless, these behaviors and transformation processes for inorganic UVFs must influence their bioavailability and toxicity, which cause great impacts on natural aquatic ecosystems [
80]. For more information, please read our full-text review.