Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Environmental
Elemental selenium (Se0) is naturally insoluble, displays a lower toxicological potential and can form selenium nanoparticles (SeNPs).
- selenite
- abiotic
- cell-free extract
- selenium nanoparticles
1. Introduction
The emergence of nanotechnology in science and technology in recent years has dramatically transformed various industries. Materials with nanometre dimensions have properties and characteristics differing from bulk materials, which open new avenues for potential applications [1]. Agricultural, material science, environmental, biomedical and other fields have all slowly become dependent on what nanotechnology has to offer. Several metal nanoparticles such as silver (Ag), gold (Au) and selenium (Se) have shown promise in the biomedical field due to their enormous potential in the delivery of drugs, proteins and genes, as well as their anti-inflammatory and antioxidant effects [2]. A recent example of advancement in nanoparticle technology is the use of gold nanoparticles for the determination of uric acid in urine samples [3]. Elemental selenium, in the form of selenium nanoparticles (SeNPs), is also one of those materials which has also garnered interest in the biomedical field. Due to their antioxidant effects and ability to be incorporated in selenoproteins, SeNPs have been used in several therapeutic applications such as cancer and diabetes [1][2]. Moreover, from concentrations as low as 1 ppm, they have also been shown to inhibit both antibiotic-resistant gram-positive and gram-negative bacteria strains [2].
In human beings, selenium concentrations of less than 55 μg·d−1 amount to selenium deficiency, whereas once it exceeds 400 μg·d−1 it can result in toxic effects [4]. Thus, the margin of safety between the essential and toxic levels of selenium is narrow, which is why minor variations in its environmental concentrations can have major impacts [2][5]. The guidelines for the maximum contaminant level for selenium in drinking water is set at 40 μg·Se/L and 50 μg·Se/L by the World Health Organization and the U.S. Environmental Protection Agency, respectively [6]. Although selenium is ubiquitous, anthropogenic activities such as phosphate mining, coal combustion and oil refining have led to selenium accumulating in both surface water and groundwater [7].
In the aquatic environment, selenium can exist in four oxidation states, namely the +6, +4, 0 and −2 states. It also occurs in organic forms [8][9]. The soluble oxyanions selenate (SeO42−) and selenite (SeO32−) are the most oxidised states of selenium. These have high solubility, which is why they are commonly found in surface waters [10]. SeO42− is the most prevalent, whereas SeO32− is the most toxic [8][11] and the most reactive, while usually being found in mildly oxidising acidic environments [9][12]. However, the oxyanions are both highly bioavailable and readily bioaccumulate in the food chain [10][13].
In contrast, elemental selenium (Se0) is naturally insoluble, displays a lower toxicological potential and can form selenium nanoparticles (SeNPs). SeNPs can be recovered and have potential uses, such as in the agricultural and pharmaceutical industries [14], for glass production and as detectors in mammographic instruments [15][16]
Several methods have been developed over the years for the remediation of selenium-laden waters. The remediation methods are in three categories, namely physical, chemical and biological techniques. Biological techniques are usually more favourable since they are deemed the most economically feasible option due to their low capital and operational and maintenance costs [17][18].
Microbial reduction can readily produce biogenic selenium nanoparticles (SeNPs) under both aerobic and anaerobic conditions. Moreover, the synthesis of SeNPs can occur at ambient temperature and pressure, making the process even more desirable. As already alluded to, microbial processes are relatively clean and eco-friendly methods. As a result, the biological synthesis of nanomaterials has drawn much attention [19]. There are a number of microbial species which are able to reduce selenite to elemental selenium as nanoparticles, but most of these utilise intracellular processes for SeNP production. Separating the intracellular nanoparticles from biomass for the purpose of selenium recovery can be achieved through processes such as cell lysis followed by filtration or centrifugation [20]. However, these processes are very energy-intensive and involve significant amounts of chemicals that can lead to further environmental contamination [21].
2. SeO32− Reduction and Selenium Nanoparticles (SeNPs) Formation
As aforementioned, reduction assays were conducted aerobically and divided into two stages, i.e., in the presence (for 1 h) and absence (for 95 h) of biomass. Figure 1 depicts the colour changes for these two stages. During the formation of red SeNPs, a garlic-like odour was generated, implying that the Enterococcus species were capable of volatilising selenium oxyanions as observed by Kagami et al. (2013) [22].
 Figure 1. SeO32− reduction at the start and end of (a) biotic stage; (b) abiotic stage.
Figure 1. SeO32− reduction at the start and end of (a) biotic stage; (b) abiotic stage.The generally observed trend was that selenite reduction was rapid for the first hour, prior to the removal of biomass. However, the percentage of selenite reduction depended on the initial selenite concentration at time 0 h. This is similar to what was observed by Dungan and Frankenberger (1998) and Tendenedzai et al. (2020) [23][24].
The selenite reduction profiles for the three concentrations are depicted in Figure 2. For the 1 mM concentration, 0.113 mM selenite was reduced during the biotic stage, translating to an average reduction rate of 0.113 mmol·(L·h)−1. A change in colour as depicted in Figure 1a corresponded with the observed reduction. For the abiotic stage, obtained results indicated that approximately 0.145 mM selenite was reduced, with an average reduction rate of 0.0015 mmol·(L·h)−1. As evident in Figure 1b, the intensity of the red colour in the MSM continued to increase, indicating the continued formation of elemental selenium and therefore that selenite reduction was still occurring in the cell-free extract. This was evident in all three concentrations.
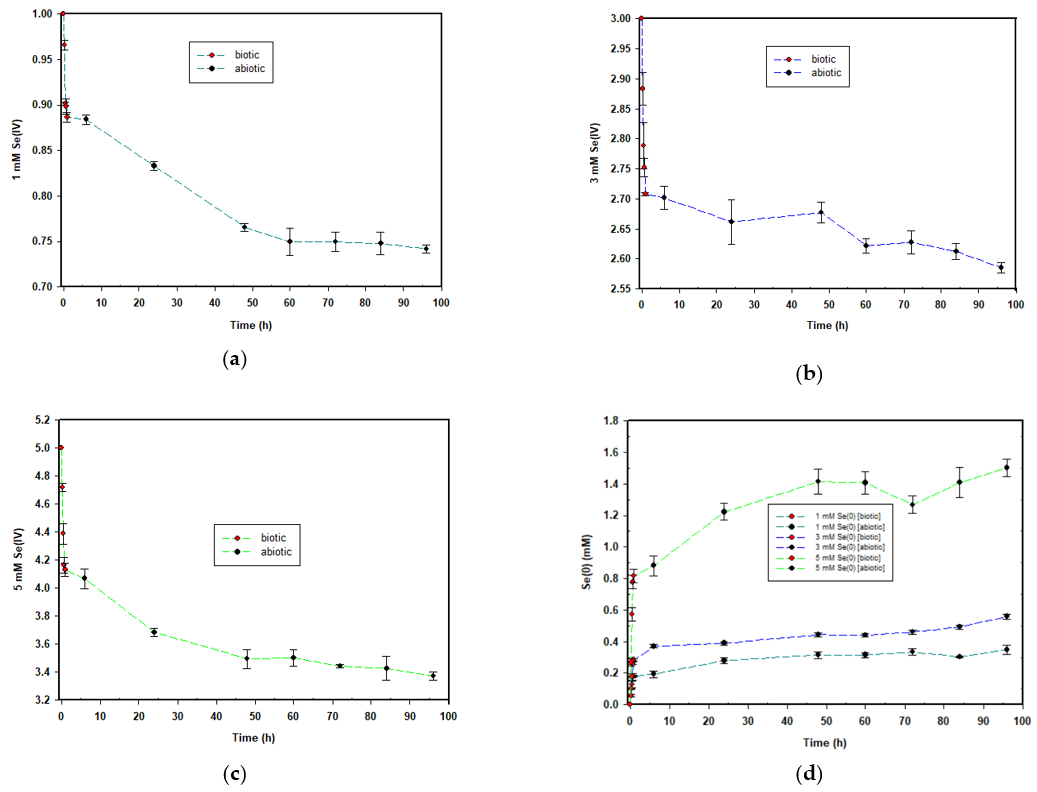 Figure 2. Time courses for the aerobic reduction of; (a) 1 mM, (b) 3 mM and (c) 5 mM SeO32− to Se0; and (d) the formation of Se0 across all the concentrations.
Figure 2. Time courses for the aerobic reduction of; (a) 1 mM, (b) 3 mM and (c) 5 mM SeO32− to Se0; and (d) the formation of Se0 across all the concentrations.For the 3 mM concentration, approximately 0.289 mM selenite was reduced during the biotic stage; the average reduction rate was 0.289 mmol·(L·h)−1. For the abiotic stage, obtained results indicated that approximately 0.192 mM selenite was reduced; the average reduction rate was 0.002 mmol·(L·h)−1.
Lastly, for the 5 mM concentration, approximately 0.868 mM selenite was reduced during the biotic stage; the average reduction rate was 0.868 mmol·(L·h)−1. For the duration of the abiotic stage, obtained results indicated that approximately 0.76 mM selenite was reduced; the reduction rate was 0.0008 mmol·(L·h)−1.
The common trend across the three selenite concentrations was that the higher the selenite concentration to be reduced, the greater the amount of selenite that was reduced, as well as the rate of selenite reduction. This is similar to what was observed in a previous study by Tendenedzai and Brink (2019) [25] in which a Pseudomonas strain was used. The explanation proposed by Tendenedzai and Brink (2019) for this observation is an increased biomass activity in response to increased selenite concentration [25]
The formation of elemental selenium nanoparticles is depicted in Figure 2d and mirrored the trend observed for selenite reduction. SeNP formation was rapid within the first hour when biomass was present, and it drastically reduced for the remainder of the 95 h during the abiotic stage.
The disproportion in the rates between the two stages described shows that the presence of biomass influences both the rate of SeO32− reduction and selenium nanoparticle formation. Moreover, it was observed that SeO32− reduction continued with the cell-free extract alone, after the biomass had been removed. This was taken as an indication of the presence of selenite-reducing biomolecules secreted into the supernatant by the bacterial cells prior to their removal. Saima Javed et al. (2015) showed that the cell-free extract of Pseudomonas pseudoalcaligenes was capable of reducing selenite. Moreover, these results were seen as confirmation that the strains released a reductase protein which reduced selenite extracellularly [26].
All three selenite concentrations showed a 48 h delay in the formation of visible SeNPs in the cell-free extract. Figure 3 shows the progression in SeNP formation. This is different when biomass is present as the formation of SeNPs is evident within the first 0.5 h [27][25].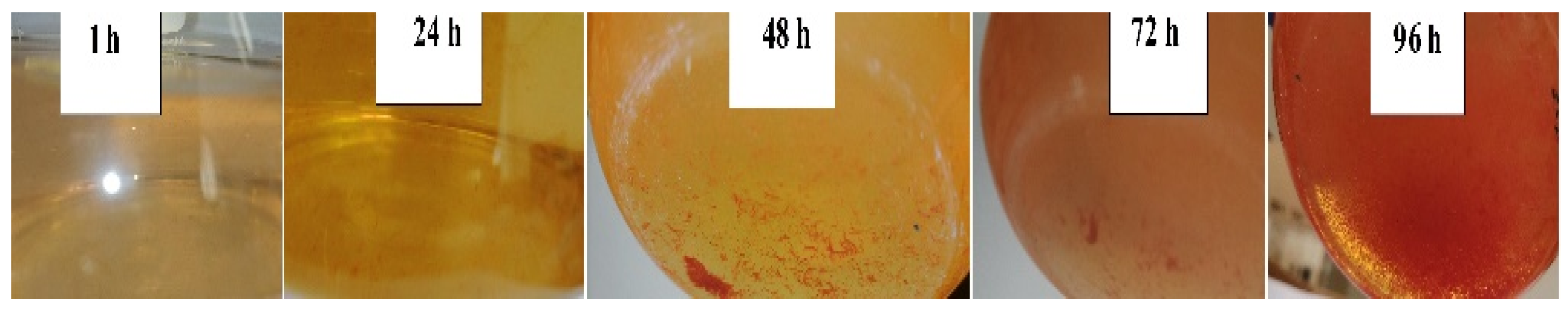

Figure 3. SeNP formation in the cell-free extract.
Table 1 summarises the average selenium balance across the three concentrations. For the 1 and 3 mM SeO32− concentrations, the total was in excess by 9% and 3%, respectively, whereas for the 5 mM SeO32− concentration, a deficit of 2.5% was observed. The excess and deficit totals were considered to be negligible and attributed to either carry-over experimental errors or the possibility that volatisation might have taken place. Some bacterial strains are known to convert selenium oxyanions into DMDSe and DMSe after prolonged incubation [22].
Table 1. Average selenium balance across the 1, 3 and 5 mM SeO32− concentrations.
| Concentration (mM) | Average SeO32− Remaining (mM) | Average Se0 Formation (mM) |
Total Average Se (mM) |
|---|---|---|---|
| 1 | 0.742 | 0.348 | 1.09 |
| 3 | 2.535 | 0.558 | 3.093 |
| 5 | 3.372 | 1.501 | 4.873 |
3. SeNP Morphology and Particle-Size Distribution
As alluded to earlier, a Zetasizer was used to analyse the particle-size distribution. The nanoparticles in each of the different concentrations were measured in order to investigate whether or not the initial concentration to be reduced had any bearings on the average particle size. The size of the Se0 particles grows until capping agents, such as proteins, polysaccharides, phospholipids or extracellular polymeric substances (EPS), inhibit further growth and aggregation [28][29]. Furthermore, proteins can restrict their size, with smaller particles observed at higher protein concentrations, while elongated incubation times can result in larger particle sizes (>200 nm) [30].
Upon analysis of the data from the Zetasizer, it became evident that different population distributions were present in each of the selenite concentrations reduced. When the frequencies were plotted, the graph was skewed to the left and thus not indicative of a normal distribution. In order to obtain a normal distribution, a log-normal distribution was applied on the particle sizes’ populations for the different concentrations.
Figure 5 shows the log-normal distribution for the 1 mM, 3 mM and 5 mM selenite concentrations. From Figure 5 can be seen that the red peaks, which are the particle sizes < 200 nm, had the highest frequency in the population regardless of the concentration. However, as the concentration to be reduced increased, the average particle sizes decreased as well. Table 2 summarises the fractions of particles in the three distinct populations, as well as the average particle sizes (d50) for the different distributions shown in Figure 5. The value for d50 defines the particle size at which the cumulative fraction of particles in a distribution reaches 50%.
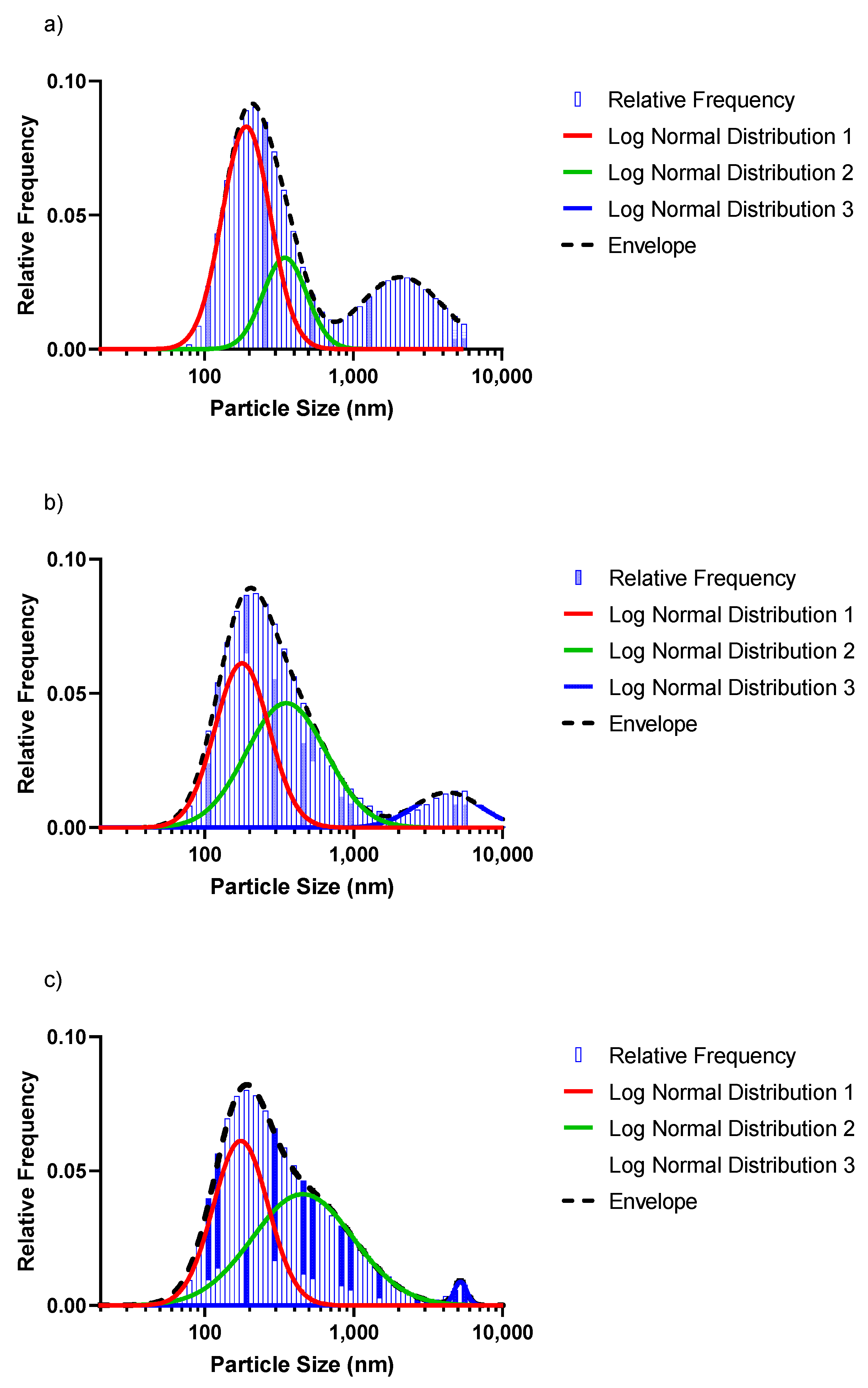 Figure 5. Log-normal distribution for the (a) 1 mM, (b) 3 mM and (c) 5 mM SeO32− concentrations, respectively.
Figure 5. Log-normal distribution for the (a) 1 mM, (b) 3 mM and (c) 5 mM SeO32− concentrations, respectively.Table 2. Distribution properties of the respective particle-size distribution of abiotically synthesised SeNPs (Figure 5).
| Selenium Concentration | Distribution 1 (Red Peak) | Distribution 2 (Green Peak) | Distribution 3 (Blue Peak) | Total Distribution (Dashed Line/Bars) | ||||
|---|---|---|---|---|---|---|---|---|
| % of Total | d50 Predicted1 |
% of Total | d50 Predicted1 |
% of Total | d50 Predicted1 |
d50 Predicted 1 |
d50 Measured 2 |
|
| 1 mM | 52.5 | 178.4 | 19.7 | 323.6 | 29.5 | 1940.1 | 267.2 | 278.8 |
| 3 mM | 43.3 | 166.6 | 48.7 | 329.4 | 10.9 | 3892.5 | 247.3 | 244.9 |
| 5 mM | 43.8 | 163.2 | 54.8 | 318.1 | 1.5 | 4846.5 | 248.5 | 246.5 |
1 As predicted from the distribution curves; 2 As measured directly by the Zetasizer.
The particles in the smaller size range (<200 nm) reduced in population (52.5% to 43.8%) and d50 (red peak) for increasing Se concentrations. The average particle sizes in the 200 nm range were 178.4, 166.6 and 163.2 nm for the 1 mM, 3 mM and 5 mM selenite concentrations, respectively. The green peak significantly increased in size, showing an increase in population fraction of 19.7%, 48.7% and 54.8% (1 mM, 3 mM and 5 mM), while the d50 remained nearly constant. The blue peaks shifted to the right, signalling an increase in the average particle sizes: 1940.1 nm (1 mM·Se), 3892.5 nm (3 mM·Se) and 4846.5 nm (5 mM·Se). In contrast, the size of the blue peak decreased significantly with a population fraction dropping from 29.5% (1 mM·Se) to only 1.5% for the 5 mM·Se. The results indicate that a significantly more homogenous size distribution (with a concomitant decreased overall d50) can be obtained with a higher Se concentration, with a concomitant decrease in average particle size.
Figure 6 shows the TEM image from the 5 mM batch and indicates that the prepared nanoparticles were mostly spherical in shape. The results from the sphericity analysis performed using ImageJ showed circularity index values of (average ± standard deviation) c = 0.88 ± 0.04, 0.75 ± 0.04 and 0.89 ± 0.01 for the 1 mM, 3 mM and 5 mM runs, respectively. The results from the circularity measurements support the observed sphericity. The reduced sphericity of the intermediate selenium concentration run (3 mM) compared to that of the other runs (1 mM and 5 mM) is not clearly understood. However, it is known that the factors affecting nanoparticle sphericity are multifactorial and integrated and therefore would involve future study to elucidate the causes thereof.
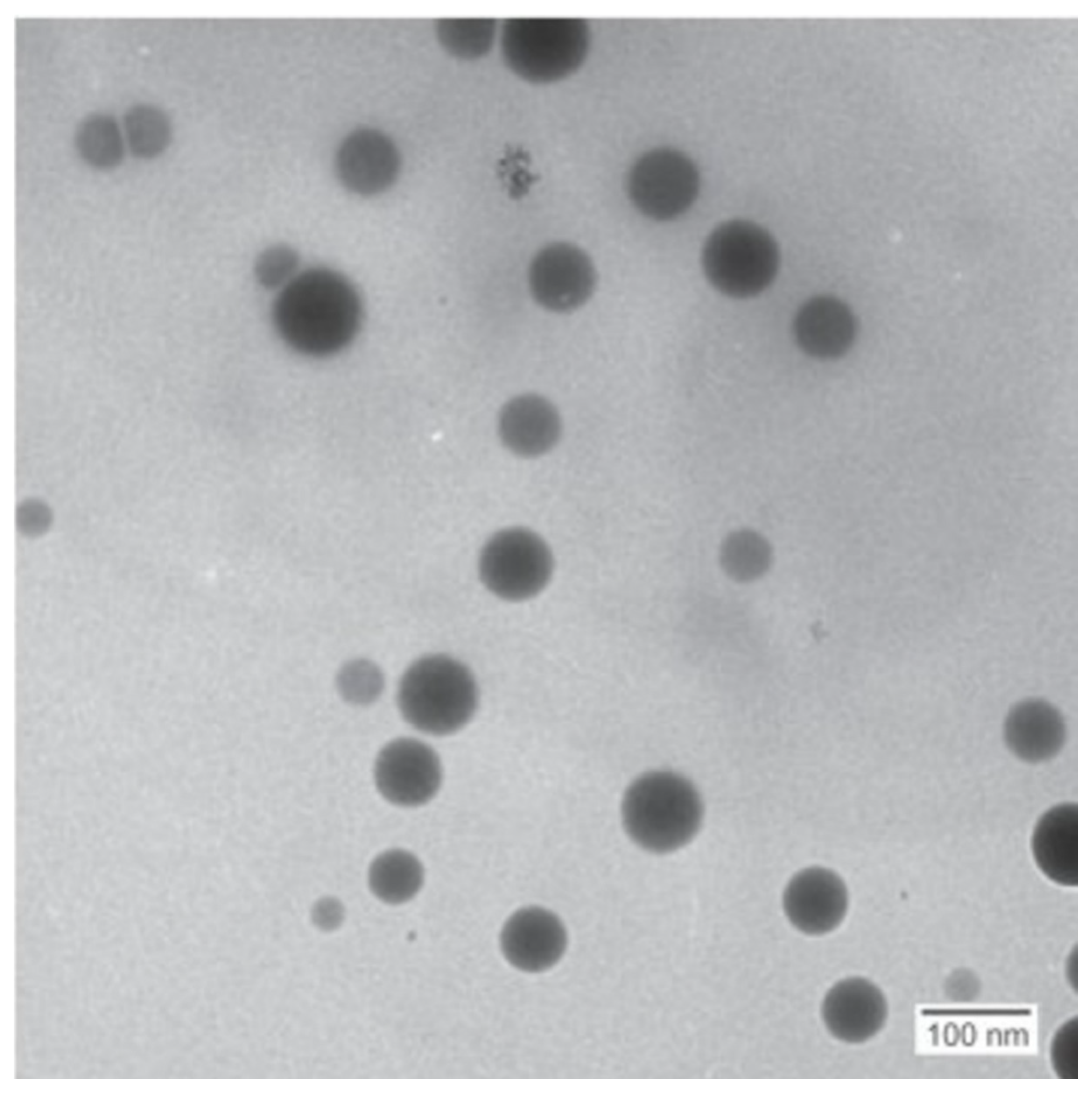 Figure 6. TEM images for SeNPs produced in the cell-free extract from the 5 mM concentration.
Figure 6. TEM images for SeNPs produced in the cell-free extract from the 5 mM concentration.This entry is adapted from the peer-reviewed paper 10.3390/nano12040658
References
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative Study of the Antimicrobial Activity of Selenium Nanoparticles With Different Surface Chemistry and Structure. Front. Bioeng. Biotechnol. 2021, 8, 1–16.
- Nayak, V.; Singh, K.R.; Singh, A.K.; Singh, R.P. Potentialities of selenium nanoparticles in biomedical science. New J. Chem. 2021, 45, 2849–2878.
- Rezaei, H.; Jouyban, A.; Rahimpour, E. Development of a new method based on gold nanoparticles for determination of uric acid in urine samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 272, 120995.
- Nancharaiah, Y.V.; Lens, P.N. Selenium biomineralization for biotechnological applications. Trends Biotechnol. 2015, 33, 323–330.
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938.
- Zhang, Z.; Chen, G.; Tang, Y. Towards selenium recovery: Biocathode induced selenate reduction to extracellular elemental selenium nanoparticles. Chem. Eng. J. 2018, 351, 1095–1103.
- Gore, F.; Fawell, J.; Bartram, J. Too much or too little? A review of the conundrum of selenium. J. Water Heal. 2009, 8, 405–416.
- Nakamaru, Y.; Tagami, K.; Uchida, S. Distribution coefficient of selenium in Japanese agricultural soils. Chemosphere 2005, 58, 1347–1354.
- Fernandez-Martinez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Bio. Technol. 2009, 8, 81–110.
- Kuroda, M.; Notaguchi, E.; Sato, A.; Yoshioka, M.; Hasegawa, A.; Kagami, T.; Narita, T.; Yamashita, M.; Sei, K.; Soda, S.; et al. Characterization of Pseudomonas stutzeri NT-I capable of removing soluble selenium from the aqueous phase under aerobic conditions. J. Biosci. Bioeng. 2011, 112, 259–264.
- Ečimović, S.; Velki, M.; Vuković, R.; Čamagajevac, I.; Petek, A.; Bošnjaković, R.; Grgić, M.; Engelmann, P.; Bodó, K.; Filipović-Marijić, V.; et al. Acute toxicity of selenate and selenite and their impacts on oxidative status, efflux pump activity, cellular and genetic parameters in earthworm Eisenia andrei. Chemosphere 2018, 212, 307–318.
- Chasteen, T.G.; Bentley, R. Biomethylation of Selenium and Tellurium: Microorganisms and Plants. Chem. Rev. 2002, 103, 1–26.
- Stewart, R.; Grosell, M.; Buchwalter, D.; Fisher, N.; Luoma, S.; Mathews, T.; Orr, P.; Wang, W.-X. Bioaccumulation and Trophic Transfer of Selenium. In Ecological Assessment of Selenium in the Aquatic Environment; Chapman, P.M., Adams, W.J., Brooks, M.L., Delos, C.G., Luoma, S.N., Maher, W.A., Ohlendorf, H.M., Presser, T.S., Shaw, D.P., Eds.; SETAC Press: Pensacola, FL, USA, 2010; pp. 93–139.
- Schrauzer, G.N. Nutritional Selenium Supplements: Product Types, Quality, and Safety. J. Am. Coll. Nutr. 2001, 20, 1–4.
- Kabir, M.Z.; Emelianova, E.V.; Arkhipov, V.I.; Yunus, M.; Kasap, S.O.; Adriaenssens, G. The effects of large signals on charge collection in radiation detectors: Application to amorphous selenium detectors. J. Appl. Phys. 2006, 99, 124501.
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016, 34, 886–907.
- Lenz, M.; Lens, P.N. The essential toxin: The changing perception of selenium in environmental sciences. Sci. Total Environ. 2009, 407, 3620–3633.
- Golder-Associates-Inc. Literature Review of Treatment Technologies to Remove Selenium from Mining Influenced Water; Golder Associates Inc.: Lakewood, CA, USA, 2009; Available online: http://www.namc.org/docs/00057713.PDF (accessed on 1 February 2022).
- Zhang, W.; Chen, Z.; Liu, H.; Zhang, L.; Gao, P.; Li, D. Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf. B Biointerfaces 2011, 88, 196–201.
- Shakibaie, M.; Khorramizadeh, M.; Faramarzi, M.A.; Sabzevari, O.; Shahverdi, A.R. Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinase-2 expression. Biotechnol. Appl. Biochem. 2010, 56, 7–15.
- Sonkusre, P. Improved Extraction of Intracellular Biogenic Selenium Nanoparticles and their Specificity for Cancer Chemoprevention. J. Nanomed. Nanotechnol. 2014, 5, 7–15.
- Kagami, T.; Narita, T.; Kuroda, M.; Notaguchi, E.; Yamashita, M.; Sei, K.; Soda, S.; Ike, M. Effective selenium volatilization under aerobic conditions and recovery from the aqueous phase by Pseudomonas stutzeri NT-I. Water Res. 2012, 47, 1361–1368.
- Dungan, R.S.; Frankenberger, W.T. Reduction of Selenite to Elemental Selenium by Enterobacter cloacae SLD1a-1. J. Environ. Qual. 1998, 27, 1301–1306.
- Tendenedzai, J.; Chirwa, E.M.; Brink, H.G. Reduction of Selenite by Use of Pseudomonas Stutzeri NT-I Cell Free Extract. Chem. Eng. Trans. 2020, 79, 373–378.
- Tendenedzai, J.T.; Brink, H.G. The Effect of Nitrogen on the Reduction of Selenite to Elemental Selenium by Pseudomonas stutzeri NT-I. Chem. Eng. Transanctions 2019, 74, 529–534.
- Javed, S.; Sarwar, A.; Tassawar, M.; Faisal, M. Conversion of selenite to elemental selenium by indigenous bacteria isolated from polluted areas. Chem. Speciat. Bioavailab. 2015, 27, 162–168.
- Tendenedzai, J.T.; Chirwa, E.M.N.; Brink, H.G. Performance Evaluation of Selenite (SeO32−) Reduction by Enterococcus spp. Catalysts 2021, 11, 1024.
- Cruz, L.Y.; Wang, D.; Liu, J. Biosynthesis of selenium nanoparticles, characterization and X-ray induced radiotherapy for the treatment of lung cancer with interstitial lung disease. J. Photochem. Photobiol. B Biol. 2018, 191, 123–127.
- Gonzalez-Gil, G.; Lens, P.N.L.; Saikaly, P. Selenite Reduction by Anaerobic Microbial Aggregates: Microbial Community Structure, and Proteins Associated to the Produced Selenium Spheres. Front. Microbiol. 2016, 7, 571.
- Zhang, J.; Wang, H.; Bao, Y.; Zhang, L. Nano red elemental selenium has no size effect in the induction of seleno-enzymes in both cultured cells and mice. Life Sci. 2004, 75, 237–244.
This entry is offline, you can click here to edit this entry!
