Trees are vital resources for economic, environmental, and industrial growth, supporting human life directly or indirectly through a wide variety of therapeutic compounds, commodities, and ecological services. Pterocarpus marsupium Roxb. (Fabaceae) is one of the most valuable multipurpose forest trees in India and Sri Lanka, as it is cultivated for quality wood as well as pharmaceutically bioactive compounds, especially from the stem bark and heartwood. However, propagation of the tree in natural conditions is difficult due to the low percentage of seed germination coupled with overexploitation of this species for its excellent multipurpose properties. This overexploitation has ultimately led to the inclusion of P. marsupium on the list of endangered plant species. However, recent developments in plant biotechnology may offer a solution to the overuse of such valuable species if such advances are accompanied by technology transfer in the developing world. Specifically, techniques in micropropagation, genetic manipulation, DNA barcoding, drug extraction, delivery, and targeting as well as standardization, are of substantial concern. To date, there are no comprehensive and detailed reviews of P. marsupium in terms of biotechnological research developments, specifically pharmacognosy, pharmacology, tissue culture, authentication of genuine species, and basic gene transfer studies.
- biotechnological tools
- DNA barcoding
- ethnomedicine
- genetic improvement
1. Introduction
2. Phytochemistry and Therapeutic Values of P. marsupium
2.1. Active Constituents
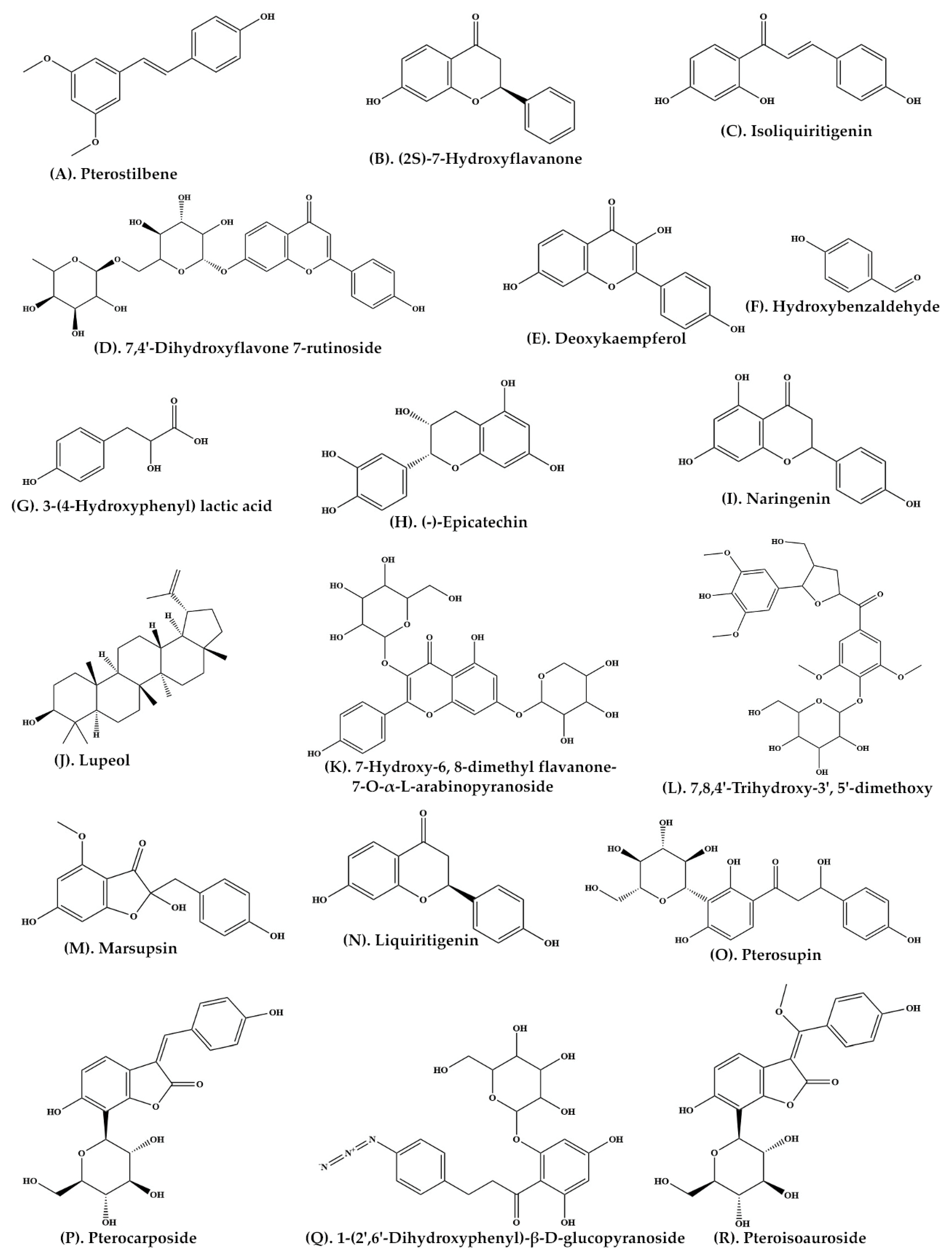
| Plant Parts | Extract Preparation |
Technique * | Bioactive Compound | References |
|---|---|---|---|---|
| Heartwood | Ethyl acetate | C-SG | Pterostilbene (Figure 2A) (2S)-7-Hydroxyflavanone (Figure 2B) Isoliquiritigenin (Figure 2C) 7,4′-Dihydroxyflavone 7-rutinoside (Figure 2D) 5-Deoxykaempferol (Figure 2E) p-Hydroxybenzaldehyde (Figure 2F) 3-(4-Hydroxyphenyl) lactic acid (Figure 2G) |
[26] |
| Bark | Ethanolic extract | C-SG | (−)-Epicatechin (Figure 2H) | [27] |
| P. marsupium extract | Ethyl acetate | C-SG | Naringenin (Figure 2I) Lupeol (Figure 2J) |
[28] |
| Roots | Ethanolic extract | C-SG | 7-Hydroxy-6, 8-dimethyl flavanone-7-O-α-L-arabinopyranoside (Figure 2K) 7,8,4′-Trihydroxy-3′, 5′-dimethoxy flavanone-4′-O-β-D-glucopyranoside (Figure 2L) |
[29] |
| Heartwood | Ethyl acetate | Thin Layer Chromatography | Marsupsin (Figure 2M) Liquiritigenin (Figure 2N) |
[23] |
| Heartwood | Ethyl acetate | C-SG | Pterosupin (Figure 2O) | [24] |
| Heartwood | Aqueous extract | C-SG | Pterocarposide (Figure 2P) | [30] |
| Heartwood | Aqueous extract | Coulman chromatography over Sephadex LH-20 | 1-(2′,6′-Dihydroxyphenyl)-β-D-glucopyranoside (Figure 2Q) | [31] |
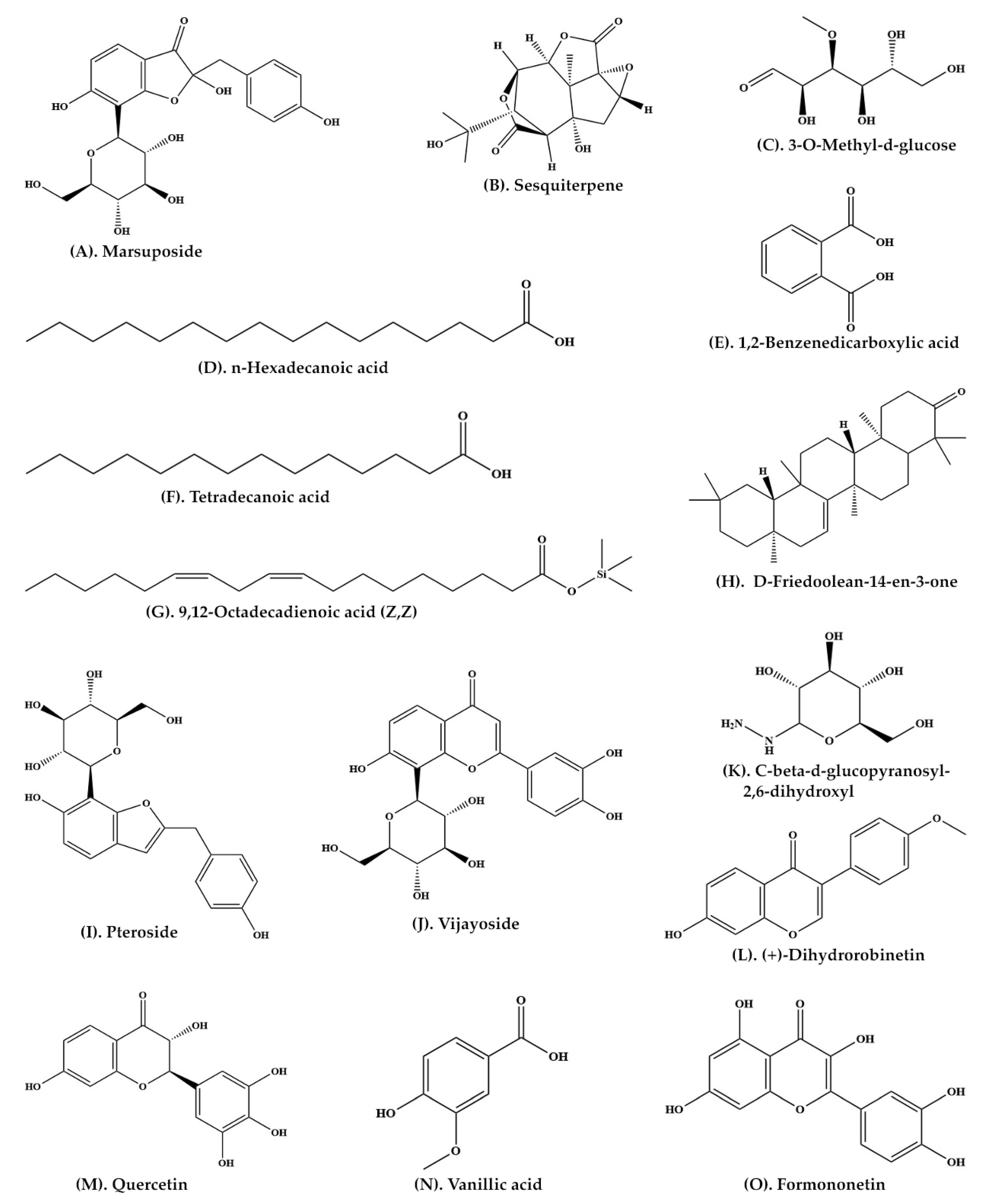
| Heartwood | Aqueous extract | C-SG | Pteroisoauroside (Figure 2R) Marsuposide (Figure 3A) Sesquiterpene (Figure 3B) |
[22] |
| Leaves | Methanolic extract | UV-spectrophotometer | Phenolics | [32] |
| Wood and bark | Ethanolic extract | GC-MS | 3-O-Methyl-d-glucose (Figure 3C) n-Hexadecanoic acid (Figure 3D) 1,2-Benzenedicarboxylic acid (Figure 3E) Tetradecanoic acid (Figure 3F) 9,12-Octadecadienoic acid (Z,Z) (Figure 3G) D-Friedoolean-14-en-3-one (Figure 3H) |
[25] |
| Apical stem bark | Methanolic extract | Followed standard protocols | Alkaloids Glycosides Flavonoids Terpenoids |
[33] |
| Heartwood | Ethanolic extract | C-SG | Pteroside (Figure 3I) Vijayoside (Figure 3J) C-β-D-Glucopyranosyl-2,6-dihydroxyl benzene (Figure 3K) |
[34] |
| Heartwood | Ethanolic extract | C-SG and HPLC | (+)-Dihydrorobinetin (Figure 3L) | [35] |
| Heartwood | Methanolic extract | LC-MS-MS | Pterosupol Quercetin (Figure 3M) Vanillic acid (Figure 3N) Formononetin (Figure 3O) |
[21] |
| Heartwood | Methanolic extract | HPLC and FTIR | Liquiritigenin | [36] |
2.2. Medicinal Properties
| S.N. | Extracts/Bioactive Compound | Potential Activities | References |
|---|---|---|---|
| 1 | (−)-Epicatechin (Figure 2H) | No effect on central nervous system Cardiac stimulant activity Anti-diabetic |
[27] |
| 2 | Flavonoids | Anti-hyperlipidemic | [23] |
| 3 | Phenolics | Anti-hyperglycemic | [24] |
| 4 | Pterostilbene (Figure 2A) | Cyclooxygenase-2 (COX-2) inhibition | [44] |
| 5 | Pterostilbene and 3,5-hydroxypterostilbene | Induce apoptosis in tumor cells | [45] |
| 6 | 5,7,2-4 tetrahydroxy isoflavone 6-6 glucoside | Cardiotonic | [37] |
| 7 | Pterostilbene | Anti-cancerous Anti-inflammatory Analgesic |
[46] |
| 8 | Phenolics | Anti-oxidant | [32] |
| 9 | Pterostilbene | Anti-cancerous Anti-proliferative |
[47] |
| 10 | Bark extract | Anti-oxidant Analgesic |
[48] |
| 11 | Extract of bark and wood | Anti-diabetic Anti-hyperlipidemic |
[49] |
| 12 | Extract of apical stem bark | Anti-microbicidal | [33] |
| 13 | Phenolic-C-glycosides | Anti-diabetic | [34] |
| 14 | Pterostilbene | Novel telomerase inhibitor | [50] |
| 15 | Heartwood extract | Dipeptidyl peptidase-4 (DPP-4) inhibition activity | [51] |
| 16 | Heartwood extract | Anti-glycation Sorbitol accumulation Inhibition of aldose reductase |
[52] |
| 17 | Pterostilbene | Inhibition of platelet aggregation | [53] |
| 18 | Heartwood extract | Reduction in body weight Anti-diabetic Anti-hyperlipidemic |
[54] |
| 19 | (+)-Dihydrorobinetin (Figure 3L) | Radical scavenging activity | [35] |
| 20 | Heartwood extract | In vitro lipid lowering activity | [21] |
| 21 | Liquiritigenin (Figure 2N) | Hypoglycemic activity | [36] |
| 22 | Pterostilbene | Sun (UV rays) protective capacity | [55] |
2.3. Micropropagation through Various Methods
3. Conclusions and Future Prospects
This entry is adapted from the peer-reviewed paper 10.3390/plants11030247
References
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 9–20.
- Klitgaard, B.; Lavin, M.D. Dalbergieae: Legumes of the world. In Legumes of the World; Lewis, G.P., Schrire, B., MacKinder, B., Lock, M., Eds.; Royal Botanic Gardens Kew: Richmond, UK, 2005; pp. 307–335.
- Baker, E.G. The Leguminosae of Tropical Africa; Erasmus Press: Ghent, Belgium, 1929.
- Bentham, G. A Synopsis of Dalbergieae: A Tribe of the Leguminosae. J. Proc. Linn. Soc. 1860, 4, 65–80.
- De Candolle, A.P. Memoires sur la Famille des Legumineuses; Auguste Belin: Paris, France, 1825.
- Lewis, G.P. Legumes of Bahia; Royal Botanic Gardens Kew: Richmond, UK, 1987.
- Rojo, J.P. Pterocarpus (Leguminosae-Papilionaceae) Revised for the World; Verlag Von J. Cramer: Lehre, Germany, 1972.
- Taubert, P. Leguminosae. In Die Natürlichen Pflanzenfamilien; Engler, A., Prantl, K., Eds.; Engelmann: Lemgo, Germany, 1894.
- Saslis-Lagoudakis, C.H.; Klitgaard, B.B.; Forest, F.; Francis, L.; Savolainen, V.; Williamson, E.M.; Hawkins, J.A. The use of phylogeny to interpret cross-cultural patterns in plant use and guide medicinal plant discovery: An example from Pterocarpus (Leguminosae). PLoS ONE 2011, 6, e22275.
- Barstow, M. Pterocarpus marsupium. The IUCN Red List of Threatened Species 2017: E. T34620A67802995; IUCN: Colombo, Sri Lanka, 2017.
- Anis, M.; Ahmad, N. Plant Tissue Culture: Propagation, Conservation and Crop Improvement; Springer: Singapore, 2016.
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitr. Cell. Dev. Biol.-Plant 2019, 55, 242–257.
- Dobránszki, J.; da Silva, J.A.T. Micropropagation of apple—A review. Biotechnol. Adv. 2010, 28, 462–488.
- Teixeira da Silva, J.A.; Kher, M.M.; Soner, D.; Nataraj, M. Red sandalwood (Pterocarpus santalinus L. f.): Biology, importance, propagation and micropropagation. J. For. Res. 2019, 30, 745–754.
- Teixeira da Silva, J.A.; Zeng, S.; Godoy-Hernández, G.; Rivera-Madrid, R.; Dobránszki, J. Bixa orellana L. (achiote) tissue culture: A review. In Vitr. Cell. Dev. Biol.-Plant 2019, 55, 231–241.
- Jiao, L.; Yu, M.; Wiedenhoeft, A.C.; He, T.; Li, J.; Liu, B.; Jiang, X.; Yin, Y. DNA Barcode Authentication and Library Development for the Wood of Six Commercial Pterocarpus Species: The Critical Role of Xylarium Specimens. Sci. Rep. 2018, 8, 1945.
- Kress, W.J. Plant DNA barcodes: Applications today and in the future. J. Syst. Evol. 2017, 55, 291–307.
- Kalimuthu, K.; Lakshmanan, K. Preliminary investigation on micropropagation of Pterocarpus marsupium Roxb. Indian J. For. 1994, 17, 192–195.
- Mishra, Y.; Rawat, R.; Nema, B.; Shirin, F. Effect of Seed Orientation and Medium Strength on In vitro Germination of Pterocarpus marsupium Roxb. Not. Sci. Biol. 2013, 5, 476–479.
- Ahmad, A. In Vitro Morphogenesis and Assessment of Genetic Diversity in Pterocarpus marsupium Roxb. Using Molecular Markers; Aligarh Muslim University: Aligarh, India, 2019.
- Singh, P.; Bajpai, V.; Gupta, A.; Gaikwad, A.N.; Maurya, R.; Kumar, B. Identification and quantification of secondary metabolites of Pterocarpus marsupium by LC–MS techniques and its in-vitro lipid lowering activity. Ind. Crops Prod. 2019, 127, 26–35.
- Maurya, R.; Singh, R.; Deepak, M.; Handa, S.S.; Yadav, P.P.; Mishra, P.K. Constituents of Pterocarpus marsupium: An ayurvedic crude drug. Phytochemistry 2004, 65, 915–920.
- Jahromi, M.A.F.; Ray, A.B.; Chansouria, J.P.N. Antihyperlipidemic Effect of Flavonoids from Pterocarpus marsupium. J. Nat. Prod. 1993, 56, 989–994.
- Manickam, M.; Ramanathan, M.; Farboodniay Jahromi, M.A.; Chansouria, J.P.N.; Ray, A.B. Antihyperglycemic Activity of Phenolics from Pterocarpus marsupium. J. Nat. Prod. 1997, 60, 609–610.
- Maruthupandian, A.; Mohan, V. GC-MS analysis of some bioactive constituents of Pterocarpus marsupium Roxb. Int. J. Chem. Tech. Res. 2011, 3, 1652–1657.
- Maurya, R.; Ray, A.; Duah, F.; Slatkin, D.; Schiff, P., Jr. Constituents of Pterocarpus marsupium. J. Nat. Prod. 1984, 47, 179–181.
- Chakravarthy, B.; Gode, K. Isolation of (-)-epicatechin from Pterocarpus marsupium and its pharmacological actions. Planta Med. 1985, 51, 56–59.
- Tripathi, J.; Joshi, T. Flavonoids from Pterocarpus marsupium. Planta Med. 1988, 54, 371–372.
- Tripathi, J.; Joshi, T. Phytochemical Investigation of Roots of Pterocarpus marsupium. Isolation and Structural Studies of Two New Flavanone Glycosides. Z. Nat. C 1988, 43, 184–186.
- Handa, S.; Singh, R.; Maurya, R.; Satti, N.; Suri, K.; Suri, O. Pterocarposide, an isoaurone C-glucoside from Pterocarpus marsupium. Tetrahedron Lett. 2000, 41, 1579–1581.
- Suri, K.; Satti, N.; Gupta, B.; Suri, O. 1-(2′, 6′-Dihydroxyphenyl)-β-glucopyranoside, a novel C-glycoside from Pterocarpus marsupium. Indian J. Chem. 2003, 42, 432–433.
- Mutharaian, N.; Sasikumar, J.M.; Pavai, P.; Bai, V.N. In vitro antioxidant activity of Pterocarpus marsupium Roxb. Leaves. Int. J. Biomed. Pharm. Sci. 2009, 3, 29–33.
- Patil, U.H.; Gaikwad, D.K. Phytochemical screening and microbicidal activity of stem bark of Pterocarpus marsupium. Int. J. Pharm. Sci. Res. 2011, 2, 36–40.
- Mishra, A.; Srivastava, R.; Srivastava, S.P.; Gautam, S.; Tamrakar, A.K.; Maurya, R.; Srivastava, A.K. Antidiabetic activity of heart wood of Pterocarpus marsupium Roxb. and analysis of phytoconstituents. Indian J. Exp. Biol. 2013, 51, 363–374.
- Deguchi, T.; Miyamoto, A.; Miyamoto, K.; Kawata-Tominaga, T.; Yoshioka, Y.; Iwaki, M.; Murata, K. Determination of (+)-Dihydrorobinetin as An Active Constituent of the Radical-Scavenging Activity of Asana (Pterocarpus marsupium) Heartwood. Nat. Prod. Commun. 2019, 14, 1–5.
- Yadav, V.K.; Mishra, A. In vitro & in silico study of hypoglycemic potential of Pterocarpus marsupium heartwood extract. Nat. Prod. Res. 2019, 33, 3298–3302.
- Mohire, N.C.; Salunkhe, V.R.; Bhise, S.B.; Yadav, A.V. Cardiotonic activity of aqueous extract of heartwood of Pterocarpus marsupium. Indian J. Exp. Biol. 2007, 45, 532–537.
- Bressers, J. Botany of Ranchi District, Bihar, India; Catholic Press: Ranchi, India, 1951; p. 96.
- Trivedi, P.C. Medicinal Plants Traditional Knowledge; I.K. International Publishing House: New Delhi, India, 2006.
- Yesodharan, K.; Sujana, K. Ethnomedicinal knowledge among Malamalasar tribe of Parambikulam wildlife sanctuary, Kerala. Indian J. Tradit. Knowl. 2007, 6, 481–485.
- Chopra, R.N.; Nayar, R.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Council of Scientific and Industrial Research: New Dehli, India, 1956; p. 78.
- Anonymous. The Wealth of India; CSIR: New Delhi, India, 1969; Volume III.
- Pullaiah, T. Medicinal Plants of Andhra Pradesh (India); Regency Publication: New Delhi, India, 1999; p. 165.
- Hougee, S.; Faber, J.; Sanders, A.; de Jong, R.B.; van den Berg, W.B.; Garssen, J.; Hoijer, M.A.; Smit, H.F. Selective COX-2 Inhibition by a Pterocarpus marsupium Extract Characterized by Pterostilbene, and its Activity in Healthy Human Volunteers. Planta Med. 2005, 71, 387–392.
- Tolomeo, M.; Grimaudo, S.; Cristina, A.D.; Roberti, M.; Pizzirani, D.; Meli, M.; Dusonchet, L.; Gebbia, N.; Abbadessa, V.; Crosta, L.; et al. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int. J. Biochem. Cell Biol. 2005, 37, 1709–1726.
- Remsberg, C.M.; Yáñez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. 2008, 22, 169–179.
- Chakraborty, A.; Gupta, N.; Ghosh, K.; Roy, P. In vitro evaluation of the cytotoxic, anti-proliferative and anti-oxidant properties of pterostilbene isolated from Pterocarpus marsupium. Toxicol. In Vitr. 2010, 24, 1215–1228.
- Tippani, R.; Vemunoori, A.K.; Yarra, R.; Nanna, R.S.; Abbagani, S.; Thammidala, C. Adventitious shoot regeneration from immature zygotic embryos of Indian Kino tree (Pterocarpus marsupium Roxb.) and genetic integrity analysis of in vitro derived plants using ISSR markers. Hortic. Environ. Biotechnol. 2013, 54, 531–537.
- Maruthupandian, A.; Mohan, V. Antidiabetic, antihyperlipidaemic and antioxidant activity of Pterocarpus marsupium Roxb. in alloxan induced diabetic rats. Int. J. Pharm. Tech. Res. 2011, 3, 1681–1687.
- Tippani, R.; Jaya Shankar Prakhya, L.; Porika, M.; Sirisha, K.; Abbagani, S.; Thammidala, C. Pterostilbene as a potential novel telomerase inhibitor: Molecular docking studies and its in vitro evaluation. Curr. Pharm. Biotechnol. 2013, 14, 1027–1035.
- Kosaraju, J.; Madhunapantula, S.V.; Chinni, S.; Khatwal, R.B.; Dubala, A.; Muthureddy Nataraj, S.K.; Basavan, D. Dipeptidyl peptidase-4 inhibition by Pterocarpus marsupium and Eugenia jambolana ameliorates streptozotocin induced Alzheimer’s disease. Behav. Brain Res. 2014, 267, 55–65.
- Gupta, P.; Jain, V.; Pareek, A.; Kumari, P.; Singh, R.; Agarwal, P.; Sharma, V. Evaluation of effect of alcoholic extract of heartwood of Pterocarpus marsupium on in vitro antioxidant, anti-glycation, sorbitol accumulation and inhibition of aldose reductase activity. J. Tradit. Complementary Med. 2017, 7, 307–314.
- Murata, K.; Deguchi, T.; Yasuda, M.; Endo, R.; Fujita, T.; Matsumura, S.; Yoshioka, Y.; Matsuda, H. Improvement of Blood Rheology by Extract of Asana, Pterocarpus marsupium-Suppression of Platelet Aggregation Activity and Pterostilbene, as a Main Stilbene in the Extract. Nat. Prod. Commun. 2017, 12, 1089–1093.
- Qadeer, F.; Abidi, A.; Fatima, F.; Rizvi, D.A. Effect of Pterocarpus marsupium in animal model of high carbohydrate diet-induced metabolic syndrome. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 1509–1514.
- Majeed, M.; Majeed, S.; Jain, R.; Mundkur, L.; Rajalakshmi, H.R.; Lad, P.; Neupane, P. A Randomized Study to Determine the Sun Protection Factor of Natural Pterostilbene from Pterocarpus marsupium. Cosmetics 2020, 7, 16.
- Gantait, S.; Kundu, S.; Das, P.K. Acacia: An exclusive survey on in vitro propagation. J. Saudi Soc. Agric. Sci. 2018, 17, 163–177.
- Fatima, N.; Anis, M. Role of growth regulators on In vitro regeneration and histological analysis in Indian ginseng (Withania somnifera L.) Dunal. Physiol. Mol. Biol. Plants 2012, 18, 59–67.
- Ahmad, N.; Javed, S.B.; Khan, M.I.; Anis, M. Rapid plant regeneration and analysis of genetic fidelity in micropropagated plants of Vitex trifolia: An important medicinal plant. Acta Physiol. Plant. 2013, 35, 2493–2500.
- Nagar, D.S.; Jha, S.K.; Jani, J. Direct adventitious shoot bud formation on hypocotyls explants in Millettia pinnata (L.) Panigrahi-a biodiesel producing medicinal tree species. Physiol. Mol. Biol. Plants 2015, 21, 287–292.
- Perveen, S.; Khanam, M.N.; Anis, M.; Atta, H.A.E. In vitro mass propagation of Murraya koenigii L. J. Appl. Res. Med. Aromat. Plants 2015, 2, 60–68.
- Howell, S.H.; Lall, S.; Che, P. Cytokinins and shoot development. Trends Plant Sci. 2003, 8, 453–459.
- Santner, A.; Estelle, M. Recent advances and emerging trends in plant hormone signalling. Nature 2009, 459, 1071–1078.
- Moubayidin, L.; Di Mambro, R.; Sabatini, S. Cytokinin–auxin crosstalk. Trends Plant Sci. 2009, 14, 557–562.
- Chandler, J.W.; Werr, W. Cytokinin–auxin crosstalk in cell type specification. Trends Plant Sci. 2015, 20, 291–300.
