The interest in the development of biobased adhesives has increased due to environmental concerns. Moreover, as the production of engineered wood products (EWPs) is expected to grow, the wood adhesives market needs to transit toward formaldehyde-free products. Cellulose nanoparticles (CNPs) are a material with unique properties and advantages for producing hybrid materials as biobased wood adhesives. Besides their traditional use as reinforcing additives, CNPs can be incorporated at the beginning of the polymerization reaction to form in situ polymerized hybrid adhesives with better mechanical and physicochemical properties than the neat adhesive.
1. Introduction
Adhesives are a preferred choice for binding solid wood and wood composites based on wood strips, chips, fibers, strands, and veneer for manufacturing engineered wood products (EWPs), such as laminated veneer lumber (LVL), laminated strand lumber (LSL), oriented strand board (OSB), cross-laminated timber (CLT), plywood, particle board, medium density fiberboard (MDF), and high-density fiberboard. An increasing population and the awareness of emitting fewer greenhouse gasses and pollutants have led to innovative programs to use wood in buildings to reduce the carbon footprint. As a result, the demand for wood-based panels in furniture and building construction is driving the market to grow. Governments such as Canada’s, for example, are developing programs that encourage the use of wood in construction project thus supporting the country’s transition to a low-carbon economy [
1]. In addition, solid wood possesses some disadvantages that limit its application, thus EWPs appear as an alternative to solid wood, providing improvement to the characteristics of the raw material [
2].
Most adhesives for manufacturing EWPs are based on formaldehyde resins, which have adverse effects on human health and the environment. The International Agency for Research on Cancer (IARC) has classified formaldehyde as a substance carcinogenic to humans [
3]. Therefore, alternative adhesives must be produced by using renewable feedstocks and novel greener processing methods, not only to reduce fossil resources but also to offer adhesives that comply with industry standards with adequate technical and mechanical properties. In the rigorous sense of the definition, biobased adhesives correspond to those adhesives produced from sources of natural, nonmineral origin that can be used as such or after small modifications [
4]. Following this definition, the renewable raw materials that have been used to synthesize biobased adhesives are lignin [
5,
6], tannin [
7,
8], carbohydrates as starch [
9,
10], and proteins [
11,
12]. Moreover, the market is driven to the development of different products, more competitive and sustainable. In these efforts, nanotechnology offers opportunities for the reinforcement of adhesives using nanocellulose mainly in the form of microfibrillated cellulose (MFC), cellulose nanofibrils (CNFs), or cellulose nanocrystals (CNCs). The unique properties of this nanomaterial, such as a high aspect ratio, crystallinity and surface area, excellent mechanical properties combined with less weight and biodegradability [
13,
14], nontoxicity, and sustainability [
15] have highlighted the benefits for the development of hybrid nanomaterials. Moreover, cellulose nanoparticles (CNPs) are recognized as reinforcing nanofillers with great potential for more sustainable polymer composite production [
16,
17]. Among the benefits of using nanocellulose as reinforcements in adhesives are the modification of the viscosity of adhesives, improvement in mechanical, physical, and thermal properties, and a reduction in formaldehyde emissions. Most of the work done in the field of CNP-based adhesives involves the mixing of polymeric matrices.
The manufacture of particleboard panels using CNFs as a sole binder met the industry requirements in terms of mechanical properties only for low-density grades [
18]. Phenol–formaldehyde (PF), urea–formaldehyde (UF) and melamine–urea–formaldehyde (MUF) adhesives are extensively used on different wood panels (laminated panels, particleboard panels, OSB, plywood panels, agglomerated panels, and so on). For example, Veigel et al. [
19] prepared particleboards and OSBs with UF and MUF in combination with CNF, resulting in a reduced thickness swelling and an improvement in the internal bond and bending strength. Bacterial cellulose (BC), untreated CNFs, and tempo-oxidized CNFs were studied by Veigel et al. [
17]. They added these biopolymers to UF adhesives showing an increase in the bond line thickness due to the high viscosity of the filled adhesive, whereas the specific fracture energy of UF-wood adhesive was improved in the case of BC and CNFs but not for modified CNFs. Zhang et al. [
20] studied modified CNCs in combination with UF resin to increase the bonding strength and reduce formaldehyde emissions. Ayrilmis et al. [
21] found that the total organic volatile compounds (TVOC) from LVLs decreased with increasing MFC on UF resin at 25 °C, which can be an environmentally friendly solution for reducing the TVOC from wood-based panels. More recently, Kawalerczyk et al. [
22] added MFC and CNCs as fillers to a UF adhesive and used it for plywood manufacturing, where they found an increase in viscosity and extension of the gel time of the adhesive and also an improvement of the mechanical properties with a slight reduction in formaldehyde emissions.
Poly(vinyl acetate) (PVA) is an alternative “green” adhesive due to its water-solubility, low toxicity, high-quality chemical resistance and biodegradability [
23]. The addition of MFC and CNFs into PVA increased thermal stability and improved shear strength on the adhesive line in tropical wood samples tested by Rigg-Aguilar et al. [
24]. Jiang et al. [
25] mixed dicarboxylic acid CNFs with commercial PVA and found an increase in the joint strength of wood joints. Moreover, Chaabouni et al. [
26] also found an increase in adhesive viscosity, the shear strength of wood joints, water resistance, and an improvement in mechanical properties when they used CNFs. In addition, Lopez-Suevos et al. [
27] mixed chemically modified CNFs with PVA to produce boards with superior heat. Kaboorani et al. [
28] have also mixed CNCs with PVA to increase the thermal stability, hardness, modulus of elasticity and creep of PVA films.
In all these examples, the CNPs were first carefully dispersed in water and then mixed with the polymers under continuous stirring, and then the mixture was cast onto the surfaces. Nevertheless, several drawbacks have been identified: (i) the process is difficult to scale-up at industrial levels; (ii) when CNPs concentration is below 1% (
w/
w) the time to achieve film formation is longer; (iii) the distribution of these nanoparticles is not homogeneous through the resulting film; and (iv) no covalent linkages are formed between the CNPs and the polymers [
29]. The nanocellulose content increases the viscosity [
22,
30,
31] and the solid content of the adhesive, limits the penetration of the adhesive, representing an obstacle for spraying and impregnation in the wood [
32], and it affects the gel time thus delaying curing [
22,
31]. New approaches should be implemented that permit nanocellulose to extend its application in wood adhesives, improving its dispersion and redistribution inside the polymer matrix, thus enhancing the interaction among nanocellulose, adhesive, and substrate [
23].
Within the constant search for better performance of adhesives, the use of nanocelluloses appears a viable option, specially by using novel techniques to polymerize in situ the resins with this biopolymer. The development of nanocellulose hybrid nanocomposites exhibits superior properties for adhesives due to the structure and surface chemistry of nanocellulose, where this solid “particle” acts as a stabilizer in heterogeneous water-based polymer systems. The ability of nanocellulose to assemble at oil–water interfaces is particularly useful and has led to hundreds of recent articles and several patents in this area [
33]. Heterogeneous (or particle forming) polymerization is an inventive technology to successfully synthetize nanocellulose-based adhesives. It consists of the combination of two (or more) immiscible liquids in which one liquid is the starting monomer that is later polymerized. Three free-radical heterogeneous polymerization categories are dispersion, suspension, and emulsion polymerization. Extensive and dedicated reviews about these types of heterogeneous polymerization systems have been done by Arshady [
34], Fritz and Olivera [
34], and Kedzior et al. [
33] and the reader is encouraged to study these publications for further investigation. The intrinsic properties of nanocellulose such as aspect ratio, surface charge density, and particle flexibility influence directly how this biopolymer stabilizes emulsion and heterogeneous water-based polymer systems [
35]. It is also critical to know the morphology of the starting nanocellulose since this can help to predict emulsion or heterogeneous water-based polymers’ stabilization mechanism [
33,
36].
Emulsion polymerization is considered as a sustainable technique to produce hybrid polymeric materials because of its use of water as the polymerization medium, it is a biobased feedstock, it prevents waste and pollution, reduces the emissions of volatile organic compounds, maximizes energy efficiency, and minimizes the potential for accidents [
37].
2. Overview of Adhesives and Adhesion Mechanism
Wood adhesives can be divided in two categories depending on their origin, natural or synthetic. Natural adhesives can be proteins of animal or vegetable origin, while synthetic ones are petroleum-based materials. At the same time, the synthetic adhesives can be separated in two types: thermoplastic adhesives and thermoset adhesives. These two types differ in their performance. The specific information about chemical structure, characteristics and implementation of these adhesives is beyond the scope of this review and the reader can find more information in several scientific reviews done by Stoeckel et al. [
38], Lengowski et al. [
2], Pizzi et al. [
4], and Forest Product Laboratory [
39]. Thermoplastic adhesives are liquid adhesives that, in general, are not as strong and stiff as wood [
39]. Thermoplastics are long-chain polymers that soften when the temperature is increased and then harden again upon cooling. Their resistance to heat, moisture, and long-term static loading is less than that of thermosetting adhesives. Common thermoplastic adhesives for wood include poly(vinyl acetate) emulsions, elastomerics, contacts, and hot-melts. In contrast, thermosetting adhesives are excellent structural adhesives that undergo irreversible chemical change when cured, forming cross-linked polymers that have high resistance to heat, moisture, and other chemicals, and can support high long-term static loads without deforming. Phenol–formaldehyde, resorcinol–formaldehyde, melamine– and melamine–urea–formaldehyde, urea–formaldehyde, isocyanate, and epoxy adhesives are examples of thermosetting polymers. Notwithstanding the large number of adhesives available for EWPs, the most used are phenol–formaldehyde, urea–formaldehyde, resorcinol–formaldehyde, and melamine–formaldehyde [
40].
Table 1 shows the adhesives used to produce EWPs, their application area, and typical uses.
Table 1. Classification of adhesives used in EWPs and their general application area (adapted from [
2,
39,
41,
42]).
| Type of Adhesive |
Adhesive |
Application Area/Typical Uses |
| Thermoplastic |
Cross-linked poly(vinyl acetate) emulsion |
Nonstructural
Interior and exterior doors; molding and architectural woodwork; cellulosic overlays |
| Polyvinyl/acrylate |
|
| Polyethylene |
|
| Polystyrene |
|
| Synthetic rubber |
|
| Thermoset |
Urea–formaldehyde |
Structural
Hardwood plywood; furniture; medium density fiberboard; particleboard; underlayment; flush doors; furniture cores |
| Melamine and melamine–urea–formaldehyde |
Structural
Melamine–urea–formaldehyde primary adhesive for durable bonds in hardwood plywood; end-jointing and edge-gluing of lumber; and scarf joining softwood plywood, ultra-low emitting formaldehyde adhesive for particleboard and fiberboard |
| Phenol–formaldehyde |
Structural
Primary adhesive for exterior softwood plywood, flakeboard, hardboard, pressed laminated wood, glued laminated wood,
waferboard and OSB and low emission particleboard |
Tannin–formaldehyde
Tannin–phenol–formaldehyde
Tannin–urea–formaldehyde |
Nonstructural
Plywood (interior and exterior) |
| Resorcinol– and phenol–resorcinol–formaldehyde |
Structural/
Primary adhesives for laminated timbers and assembly joints that must withstand severe service conditions |
| Isocyanate-based adhesives |
Structural
Laminated strand lumber, OSB, I-beams |
The most used thermosetting wood adhesive worldwide is UF with approximately 11 million tons per year, which is mainly utilized for wood panel composites for the preparation of interior furniture and panels [
4]. Although it lacks resistance to exterior weather conditions and formaldehyde emissions, its full substitution is difficult to achieve because of its low cost, low cure temperature, short pressing time, excellent adhesive performance, and ease of handling. MUF adhesives can be considered as an improved version of traditional melamine–formaldehyde ones, since the expensive melamine resin is partially replaced with urea. MUF adhesives exhibit an enhancement in water and weather resistance, mechanical strength, and a diminution of formaldehyde emission. MUFs exhibit a medium price, cure temperature and pressing time. PF adhesives are the second most important wood composite adhesive, with 3 million tons per year used worldwide [
4]. PFs have a medium price, high cure temperature, medium pressing time, and very low formaldehyde emissions [
43]. In contrast, phenol–resorcinol–formaldehyde adhesives are cold-setting adhesives. These adhesives are expensive due to the high cost of resorcinol; however, they are binders for fully exterior-grade and weather-resistant composites, with a low volume of around 30 thousand tons per year worldwide [
4].
Understanding wood adhesion theories and mechanisms would enable the production of adhesive formulations suitable for a wide range of applications of EWPs and would encourage the development of novel biopolymers-based adhesives. The role of an adhesive for wood is to transfer and distribute loads between components, thereby increasing the strength and stiffness of wood products [
42].
There is no universal theory of adhesion on which to accurately model all interactions that take place between the adhesive and the adherend; nor is there an agreement about the mechanisms involved. The existing theories of adhesion are generally useful in understanding why and how adhesives stick and why they fail. The adhesion theories provide methods to rationalize and to make predictions about the joint strength. Being familiar with these theories and mechanisms would allow the development of an understanding and awareness of adhesives’ performance. An entirely satisfactory definition of adhesion has not been established yet; however, according to the American Society for Testing and Materials (ASTM) [
44], adhesion corresponds to the state in which two surfaces are held together by interphase forces. Wu [
45] defined that those forces arise from van der Waals forces, chemical bonding, or electrostatic attraction.
Traditionally, mechanical interlocking, adsorption/specific, electrostatic, and diffusion adhesion theories have been applied to define the mechanisms of adhesion. However, other theories have arisen to explain adhesive bonding mechanisms, such as wettability, covalent chemical bonding, acid–base, and weak boundary layers theories. A combination of different theories would explain the complex adhesive mechanism since it is often difficult to fully attribute it to one individual theory.
The mechanical theory proposes that mechanical, physical, and interlocking of an adhesive into the macro- and microirregularities of the substrate’s surface is the major factor of adhesion. The adhesion occurs when an adhesive penetrates the porous wood surface, displacing the trapped air at the interface. If an adhesive flows deeply into cell cavities, the mechanical interlocking increases thus increasing the bonding [
46].
The specific adhesion between the adhesive and the adherend involves the bond created because of molecular attraction between the surfaces in contact. This theory is the most widely accepted, and an applicable theory of adhesion. The intermolecular attractive forces that participate in the specific adhesion can be ionic, covalent, or induced by any other intermolecular forces. Therefore, ionic interactions or hydrogen bonds are due to strong dipole–dipole forces; van de Waals forces are due to a fixed dipole in one molecule that induces oscillating dipoles in another molecule. These electrodynamic forces can be divided as Keesom (permanent–permanent dipoles) interaction; Debye (permanent–induced dipoles) forces; and London dispersion forces (fluctuating dipole–induced dipole interaction). The strength of these van de Waals interactions is strongly dependent on distance, decreasing with the sixth power of the interatomic or molecular distance.
Kumar and Pizzi [
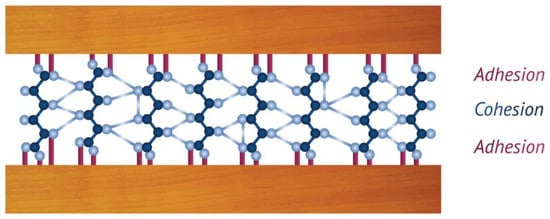
42] explained the mechanism of adhesion for wood by adding another type of adhesion called effective adhesion, which combines the specific and mechanical adhesion explained above. They also established that the bonding effectiveness is a combination of adhesion and cohesive strength. Cohesion is defined as the internal strength of an adhesive because of a variety of interactions within the adhesive (
Figure 1). In this way, an adhesive bond would fail if either an interfacial adhesion failure occurs (if the adhesive is separated from substrate) or if there is a cohesive failure.
Figure 1. Adhesion and cohesion forces on wood surfaces explained by chemical bonds and intermolecular forces [
47].
The formation of an electrical double layer at the adhesive–adherend interface is explained by the electrostatic theory. These forces are primarily dispersion forces and forces arising from the interaction of permanent dipoles. The electrostatic theory is often used to describe the adhesion behavior of powders to solid surfaces [
48,
49,
50]. Practical applications of this theory to wood and EWPs is limited to coatings of furniture, sandpaper manufacture, inkjet printing, novel bioactive papers, and colloidal interactions in papermaking [
51,
52,
53,
54].
In the case of wood surfaces, the diffusion theory is unlikely to explain the adhesive mechanism since both the adhesive and adherends should be long-chain polymers capable of movement and exhibit similar values of solubility [
42,
55]. Wood is heterogeneous in composition, the basic three biopolymers, cellulose, lignin, and hemicellulose, exhibit different chain lengths, molecular weight, and crystallinity. However, in the case of wood adhesive bonding, this theory is applicable if the adhesive can diffuse or penetrate into the cell wall, which is not the case for thermosetting wood adhesives [
56].
The wetting theory proposes that adhesion results from molecular contact between two materials and the surface forces that develop. For an adhesive to wet a solid surface, the adhesive should have a lower surface tension than the critical surface tension of the solid [
55]. Incomplete wetting generates interfacial defects, thereby reducing the adhesive bond strength. Complete wetting achieves the highest bond strength. Hiziroglu et al. [
57] demonstrated that roughness of wood surfaces affected their wettability, thus affecting the strength of bonding.
Covalent bonds occur in certain fields of adhesion; however, their existence was for a long time not believed to occur between wood and adhesives [
58,
59]. Zhou and Frazer [
60] and Das et al. [
61] studied phenyl isocyanate-based adhesives and determined that these adhesives are likely to form urethane (or carbamate) bonds with wood biopolymers, also showing these adhesives can penetrate the wood cell wall and intimately associate with wood biopolymers. Gardner et al. [
62] concluded that it is very likely that covalent bonds between the wood and adhesive are not necessary for durable wood adhesive bonds. This is because the contribution of the formation of adhesive–substrate covalent bonds induced by lignin [
63] is very small and often negligible under the conditions pertaining to thermosetting adhesive applications [
63,
64] and do not exist for MUF systems [
65].
According to the acid–base theory, adhesion results from the polar attraction of Lewis acids and bases. It has been identified that hydrogen bonding is a special type of acid–base interaction. In addition, in chemically heterogeneous materials such as wood, the extractives are the dominant factor influencing the acid–base characteristic [
66].
The weak boundary layer theory establishes that bond failure at the interface is caused by either a cohesive break or a weak boundary layer [
67]. Weak boundary layers can originate from the adhesive, the adherend, the environment, or a combination of any of these three factors. Here, the wetting of surfaces is key, since if the adhesive does not wet the substrate, a weak boundary layer is formed at the interface, causing a reduction in joint strength.
This entry is adapted from the peer-reviewed paper 10.3390/polysaccharides3010012