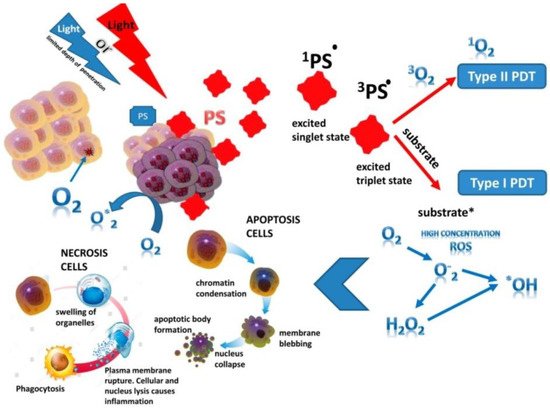
2. The Meaning and Mechanism of Cell Damage during Photodynamic Therapy
Photoreactions. The PS singlet state is characterized by the presence of two low-energy electrons with opposite spins on the molecular orbital. After absorbing of photons emitted by a specific wave of light, one of the electrons moves to a higher energy orbital while maintaining its spin (the first excited singlet state) (
Figure 1). The electron at this state exist for nanoseconds and loses its energy, emitting light (fluorescence) or converting it into heat. The excited singlet state PS is characterized by the fact that the spin of the activated electron is inverted into the triplet state for a relatively long period (from microseconds to milliseconds), in which both electron spins are parallel–intersystem crossing [
9].
Figure 1. The principle of photodynamic reaction. PS—photosensitizer (*: activated state).
The excited PS triplet can interact with molecules according to Jablonski by three types of reactions. At I type reactions, the triplet PS can receive an electron from a nearby reducing agent. For example, it is a nicotinamide adenine dinucleotide (NADH) molecule or reduced NAD phosphate (NADPH) in cells. In this case, PS is a radical anion that does not have an additional unpaired electron (PS−•). In another case, two triplet PS molecules as radical cation and anion can interact with each other with intermolecular electron transfer. The radical anion forms PS can react with oxygen, which results in electron transfer and the formation of reactive oxygen species (ROS), in particular superoxide anion (Figure 1).
In II type reaction, the PS triplet transfers its energy directly to molecular oxygen and singlet oxygen is formed in an excited state (
Figure 1). In this reaction, PSs retain their molecular structure in a multiple photoactivated state. In some cases, one PS molecule can generate 10,000 singlet oxygen molecules. Singlet oxygen, formed during the reactions of type II, is considered the most important molecule responsible for cell damage during PDT [
10,
11,
12]. However, due to the high reactivity and short half-life of singlet oxygen, only the molecules and structures located near the region of its localization are influenced by PS. The least common type of reaction is III, where PS in the triplet state directly reacts with the biomolecule and only they are simultaneously destroyed, without the participation of oxygen. It should be noted that reactions of types I and II can occur simultaneously, and the ratio between them depends on the type of PS used, the concentration of the substrate and oxygen and its active forms (singlet oxygen, O
2•, H
2O
2•; OH
• and NO). The aforementioned ROS are oxidizing agents that can directly react with amino acid residues in proteins, including cysteine, methionine, tyrosine, histidine, and tryptophan. These molecules were found in cells and tissues after PDT [
13,
14], which indicates of their decisive role in PS-induced cytotoxic effects [
15,
16]. If PS is not localized in the cell, its photodynamic activity is relatively low. The efficiency of photosensitization is determined by the quantum yield of the formation of the triplet state of oxygen molecules. In this case, the chemical transformation of the PS does not occur, and after the transfer of the excitation energy to molecular oxygen, it returns to the ground stable state, and the whole cycle can be restarted after the absorption of a new quantum of light energy [
17].
Antitumor effects of PDT on cells. The phototoxic effect of PS on tumor cells is realized by their direct destruction upon interaction with an organic molecule, which acquires a hydrogen atom or an electron. As a result, a superoxide anion radical is generated in type I photoreaction, or by indirect interaction with molecular oxygen also with the formation of singlet oxygen in photoreaction II type. The ratio of reactions of type I and type II in the tissues of the organism depends on the structure of the PS, the substrate, the concentration of oxygen and the affinity of its binding to the substrate [
2,
4,
15]. Type II reaction prevails during PDT; in this case, singlet oxygen is the main cytotoxic agent inducing biological effects [
13]. Intracellular targets of PS are mitochondria, endoplasmic reticulum, lysosomes, the Golgi apparatus, and the plasma membrane of cells. The generation ROS in photodamage of cellular structures leading to cell death (
Figure 1). Most PSs are not localized in the nucleus; therefore, PDT does not cause DNA damage, mutations, and carcinogenesis. It is noted that the probability of cell death depends on the lipophilic properties of PS and of the hydrophilic drugs, which is much lower and thus indicates the decisive role of membrane structures in cell damage [
18]. So, it has been shown that PS activation with the generation of hydroxyl radicals in the endoplasmic reticulum is a more effective strategy for enhancing the therapeutic effect of PDT than its lysosomal localization with the production of hydrogen peroxide in lysosomes [
19]. The most active of the PSs have low toxicity in the dark and high phototoxicity under irradiation [
20]. In addition to separating PSs by types of photochemical mechanism (type I or II), they are also distinguished by their properties to localize in cell organelles (lysosomes, endoplasmic reticulum, mitochondria, etc.). So, hydrophobic PSs are localized to a greater extent in mitochondria and endoplasmic reticulum due to the large proportion of lipid bilayers in these organelles, which is much more likely to cause apoptosis. The phototoxic effect of such PSs uses more type I photochemistry and create hydroxyl radicals when surrounded by an aqueous environment, whereas hydrophilic PSs are localized mainly in lysosomes and undergo type II photochemical reactions, inducing the formation of a large amount of singlet oxygen [
21]. In 2019, a study by Baptista [
22] radically changed the idea that PS are active only when they are localized in the cell. The fundamental role of contact-dependent reactions, which usually cause PS photobleaching and irreversible damage to biological membranes, has been shown. Thus, Mg (II) porphyrazines (MgPzs), which have similar quantum yields of singlet oxygen and side groups with different electron-withdrawing strengths, have different redox properties. The process of their photobleaching is based on the photoinduced detachment of electrons from the surrounding electron-rich molecules (solvent or lipid molecules) and the formation of a radical anion. The photobleaching of MgPzs porphyrazines depends on the degree of lipid unsaturation due to the detachment of electrons from the lipid double bond when incorporated into phospholipid membranes. A high rate of induced leakage in membranes corresponds to a high rate of photobleaching. The results obtained have a major impact on further strategies for the development of new photosensitizers.
In PDT, the death of tumor cells occurs in a programed (apoptosis) or unprogramed (necrosis) way [
23]. At high light intensity, tumor cells die through necrosis, which is characterized by vacuolization of the cytoplasm and destruction of the cell membrane (
Figure 1). In this case, a local inflammatory reaction occurs in response to the appearance of cellular debris and pro-inflammatory mediators in the extracellular space [
24]. Low doses of light during PDT initiate the genetically encoded cell death, or apoptosis [
22,
25]. During this process, the structure of cells changes, nuclear chromatin condenses, and chromosomal DNA is cleaved into internucleosomal fragments (
Figure 1). At this time, cells decrease in size and apoptotic bodies are formed, surrounded by a plasma membrane [
26]. This type of cell death does not induce an immune response, since cellular debris does not appear in the extracellular space [
22,
27]. Apoptosis can turn into necrosis with an excessive decrease in the availability of caspases and the intracellular concentration of adenosine triphosphate (ATP) [
28]. Additionally, when apoptosis is disturbed, another mechanism of cell damage is paraptosis with the photodamage of the endoplasmic reticulum during PDT [
29]. Paraptosis proceeds independently of the activation and inhibition of caspases, without condensation of chromatin and fragmentation of the nucleus with the vacuolization of the cytoplasm in contrast to apoptosis.
The Photodynamic Processes. The success of PDT is based on PSs’ ability to penetrate into the target tumor with minimal damaging effect on the healthy tissues of organism. In cancer therapy, depending on the localization of the tumor, PS can be administered intravenously, orally, or locally. ROS is generated at the site of the localization of the PS where it is irradiated with a light source of the appropriate wavelength, which indicated the death of cancer cells without affecting healthy tissues. The maximum concentration of PS is reached in tissues after 24–72 h. The direct destruction of tumor cells with the using of PS also leads to the destruction of tumor microvessels, since endothelial cells can concentrate PS, initiating the production ROS that is activated by the appropriate light. The violation of the vascular walls (vascular effect) during PDT leads to the cessation of access to the tumor of oxygen and nutrients, which indicates the long-term effectiveness of such a therapy [
24]. The effectiveness of the vascular effect of PDT is achieved by using a short interval between the systemic injection of PS with its localization in the vascular network and the precise effect of radiation on the tumor [
27].
In addition to direct and vascular effects, PDT can significantly affect the adaptive immune response by stimulating or suppressing it. Its effectiveness depends on the degree of antitumor immunity induction. So, if the long-term control of a tumor with a combination of direct and vascular effects of PS activates the immune response [
30], then the immunosuppressive effect of PDT is mainly associated with reactions to local treatment with a high flow rate and over large areas of irradiation [
24]. Changes in tissue integrity and homeostasis with cell necrosis induce an acute inflammatory response due to oxidative damage to the tumor stroma during PDT, initiated by the release of pro-inflammatory mediators, including various cytokines, growth factors, and proteins [
24,
25]. Innate immune cells (neutrophils, mast cells, macrophages and dendritic cells) at the site of injury phagocytose the breakdown products of cancer cells and provide proteins to the helper CD4
+ T lymphocytes [
31]. The cytotoxic T cells can recognize and specifically destroy tumor cells and can circulate throughout the body for a long time, providing a systemic anti-tumor immune response. After several cycles of absorption, PS can degrade and lose the ability to trigger a photodynamic reaction; in this case, the process of its burnout is called photobleaching [
32]. On the other hand, the insufficient penetration depth of laser light (4–8 mm depending on the wavelength), lack of a reliable evidence base, difficulties in planning, dosimetry and monitoring of processes due to the complex interaction of photons with biological tissue are restrictions to the application of PDT [
33].