Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Traditionally, neutrophils were seen as terminally differentiated cells destined to commit suicide on their one-way mission from bone marrow to the tissue. Neutrophils are an essential component of the innate immune response, but they are also a major contributor to inflammation. Neutrophil homeostasis is tightly regulated through balance between granulopoiesis, bone marrow storage and release, intravascular margination, and clearance of dying cells.
- neutrophils
- inflammation
- cell death
1. Apoptosis
Apoptosis represents a conserved mechanism of programmed cell death [1]. Neutrophils die by constitutive apoptosis, an essential mechanism for neutrophil functional shutdown regulation [2][1]. Apoptotic neutrophils are characterised by a series of typical morphological features such as cytoplasmic shrinkage, nuclear condensation, DNA fragmentation and membrane blebbing [3]. They must display engulfment signals, known as “find-me” and “eat me” signals on their plasma membranes, such as the expression of today most studied phosphatidylserine receptor or calreticulin [4][5][3], which permit recognition and phagocytic engulfment of dying cells by macrophages (efferocytosis) [4]. Apoptosis depends on the balance between pro- (e.g., BAX, BAK) and anti-apoptotic (e.g., BCL-2, BCL-B, BCL-xL, BCL-W, MCL-1, A1) factors, which are both members of the B-cell lymphoma-2 (BCL-2) family of proteins [6]. The anti-apoptotic protein myeloid cell leukemia sequence 1 (MCL-1) plays a key role in the regulation of neutrophil apoptosis [3][7][8]. Important to note, cell cycle regulatory proteins, that in other cell types serve to control proliferation, in neutrophils, surprisingly, regulate apoptosis and survival [1][9]. Thus, as neutrophil-specific regulatory factors, proliferating cell nuclear antigen (PCNA), myeloid nuclear differentiation antigen (MNDA) and cyclin-dependent kinases (CDKs) are identified [1][9]. These neutrophil-specific regulatory proteins are localized in the nucleus of proliferating cells, while in mature neutrophils they are located in the cytoplasm where they act as pro-survival (PCNA, CDKs) or pro-apoptotic (MNDA) factors [1][9]. Neutrophil apoptosis is usually initiated by the intrinsic or extrinsic apoptotic pathway [10]. The intrinsic pathway mediates constitutive neutrophil apoptosis, which is driven by the permeabilisation of the mitochondrial outer membrane and subsequent release of cytohrome c from mitochondria, which leads to the activation of caspase-9, which in turn causes the activation of frequently activated death protease, caspase-3 [4][11]. The extrinsic pathway of neutrophil apoptosis is induced by ligation of TNF receptor superfamily cell surface death receptors, which drives caspase-8 dependent activation of caspase-3 [4][3].
Neutrophil apoptosis represents a pro-resolution mechanism that limits the extent of inflammation and consequently tissue injury, since efferocytosis occurs before the plasma membrane of apoptotic cells becomes leaky [3]. Therefore, whereas nonlytic apoptotic cell death allows controlled removal of cells and promote healing and anti-inflammatory responses, pathways ending with loss of cell membrane integrity, such as NETotic death, necrosis and necroptosis, results in leakage of pro-inflammatory and toxic cell contents into the extracellular space and may elicit deleterious proinflammatory responses [12][13] (Figure 1B). More importantly, dysregulated apoptotic neutrophil death, either upregulated or downregulated, is often linked solely to chronic inflammatory diseases like cancer [13] or autoimmune diseases [6][14]. Reduced neutrophil apoptosis supports inflammation, stroma remodeling, tumor angiogenesis and metastasis [13][14].
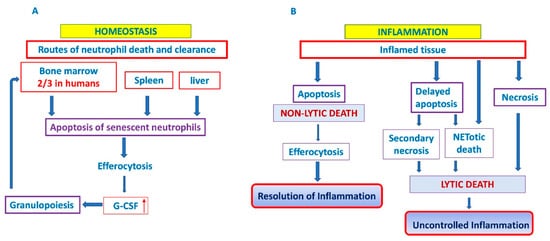
Figure 1. Neutrophil cell death and clearance. In homeostatic conditions, clearance of aged neutrophils is believed to occur via their uptake into the bone marrow (predominantly in humans), liver and spleen. It generates homeostatic signals leading to granulocyte colony-stimulating factor (G-SCF) release that drives granulopoiesis as a positive feedback loop (A). In inflammatory conditions, two outcomes are possible: efferocytosis of apoptotic neutrophils and resolution of inflammation or delayed apoptosis and progression towards lytic cell death and uncontrolled inflammation (B). G-CSF: granylocyte colony stimulating factor. ↑ denotes increased expression.
2. Clearance of Apoptotic Neutrophils
In the absence of an infectious challenge, homeostatic clearance of aged neutrophils is believed to occur via their uptake into the liver, spleen and bone marrow, where they are phagocytosed by tissue-resident macrophages and dendritic cells (DC) [15][9][16]. In a steady state, about a third of all circulating senescent neutrophils in mice are phagocytosed in the bone marrow resident macrophages [17], while the level of phagocytosed neutrophils in the human bone marrow can go as high as 67% (two-thirds) of circulating neutrophils [16]. Thus, bone marrow is the place of ultimate neutrophil production [18], and a site of their homeostatic clearance [16][19][20] (Figure 1A).
Clearance of peripheral blood senescent neutrophils, that express chemokine receptor CXCR4 and home back to the bone marrow [21][22] generates homeostatic signals leading to granulocyte colony-stimulating factor (G-SCF) release that drives granulopoiesis as a positive feedback loop for homeostatic regulation of circulating neutrophils [21][23]. This process is closely regulated in both mice and humans [21][22]. A mechanism that regulates neutrophil production and completes feedback loop responsible for the tight control of neutrophil number in the peripheral tissue and in the circulation was demonstrated in in vitro and complementary in vivo studies by Stark et al. [21], and involves the IL-23/IL-17/G-CSF feedback circuit. Phagocytosis of apoptotic neutrophils by tissue dendritic cells and macrophages reduces the production of IL-23, which in turn reduces the production of IL-17, leading to less G-CSF and reduced proliferation, and differentiation of neutrophil precursors in the bone marrow [21]. Conversely, when neutrophil migration into tissue is blocked by adhesion molecules deficiency, the result of this event is decreased phagocytosis, increased IL-23, IL-17 and G-CSF, and the production of neutrophils is enhanced [21][24][23].
In the case of tissue injury and/or infection neutrophils follow the gradient of chemoattractants, such as CXCL8 (IL-8) that determines the direction of neutrophil migration into the tissues [13][9]. After fulfilling their biological function (elimination of pathogens by phagocytosis, degranulation, release of ROS or formation of NETs, etc.), neutrophils are removed in situ by macrophages and by dendritic cells [5]. A portion of neutrophils migrates back to the vasculature, then they reverse migrate to marrow, passing through to the lungs en route to the bone marrow, where they undergo apoptosis [25]. Phagocytosis of apoptotic neutrophils by macrophages is not just dead cell disposal system because tissue phagocytes provide a mechanism for the safe disposal of apoptotic material. Signaling pathways will regulate the phagocytic response and determine whether apoptotic cell clearance is immunologically “silent” or even anti-inflammatory [26]. It suppresses the production of G-CSF to limit the inflammation [21][24] and reduces the number of viable and activated neutrophils without releasing the potentially harmful enzymes and ROS, thereby facilitating the resolution of inflammatory response. Furthermore, efferocytosis has an important influence on the resolution of inflammation, through secretion of anti-inflammatory cytokines, such as TGF-β, IL-10 and VEGF, by macrophages [27][28]. On the other hand, an elevated level of TGF-β could drive unrestrained fibrotic responses, like in MPN [5][29]. Neutrophil longevity during inflammation may be extended by various stimuli, including pattern recognition signals, growth factors (G-CSF, GM-CSF) or chemokines [24][4][5]. As mentioned above, delayed death and clearance of neutrophils could progress either to uncontrolled inflammation of related tissue [30] or toward lytic cell death, such as secondary necrosis [4][5][3][31], or NETotic death [32], which usually triggers inflammation in both cases [14][31]. Thus, it has been shown that macrophages produce the proinflammatory cytokines and chemokines, such as TNF-α and IL-8, largely due to proteases liberated by lysed neutrophils [33].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031490
References
- McCracken, J.M.; Allen, L.-A.H. Regulation of Human Neutrophil Apoptosis and Lifespan in Health and Disease. J. Cell Death 2014, 7, 15–23.
- Simon, H.-U. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol. Rev. 2003, 193, 101–110.
- Vermeren, S.; Karmakar, U.; Rossi, A.G. Immune complex-induced neutrophil functions: A focus on cell death. Eur. J. Clin. Investig. 2018, 48, e12948.
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357–370.
- Bratton, D.L.; Henson, P.M. Neutrophil clearance: When the party is over, clean-up begins. Trends Immunol. 2011, 32, 350–357.
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193.
- Moulding, D.; Akgul, C.; Derouet, M.; White, M.; Edwards, S.W. BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis. J. Leukoc. Biol. 2001, 70, 783–792.
- Edwards, S.; Derouet, M.; Howse, M.; Moots, R. Regulation of neutrophil apoptosis by Mcl-1. Biochem. Soc. Trans. 2004, 32, 489–492.
- Witko-Sarsat, V.; Pederzoli-Ribeil, M.; Hirsh, E.; Sozzani, S.; Cassatella, M.A. Regulating neutrophil apoptosis: New players enter the game. Trends Immunol. 2011, 32, 117–124.
- Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Relevance of granulocyte apoptosis to resolution of inflammation at the respiratory mucosa. Mucosal Immunol. 2008, 1, 350–363.
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104.
- Lawrence, S.M.; Corriden, R.; Nizet, V. How Neutrophils Meet Their End. Trends Immunol. 2020, 41, 531–544.
- Brostjan, C.; Oehler, R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 2020, 6, 26.
- Anderton, H.; Wicks, I.P.; Silke, J. Cell death in chronic inflammation: Breaking the cycle to treat rheumatic disease. Nat. Rev. Rheumatol. 2020, 16, 496–513.
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324.
- Szczepura, K.R.; Ruparelia, P.; Solanki, C.K.; Balan, K.; Newbold, P.; Summers, C.; Chilvers, E.R.; Peters, A.M. Measuring whole-body neutrophil redistribution using a dedicated whole-body counter and ultra-low doses of 111Indium. Eur. J. Clin. Investig. 2010, 41, 77–83.
- Furze, R.C.; Rankin, S.M. Neutrophil mobilization and clearance in the bone marrow. Immunology 2008, 125, 281–288.
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000, 404, 193–197.
- Dresch, C.; Flandrin, G.; Breton-Gorius, J. Phagocytosis of neutrophil polymorphonuclears by macrophages in human bone marrow: Importance in granulopoiesis. J. Clin. Pathol. 1980, 33, 1110–1113.
- Saverymuttu, S.H.; Peters, A.M.; Keshavarzian, A.; Reavy, H.J.; Lavender, J.P. The kinetics of 111Indium distribution following injection of 111Indium labelled autologous granulocytes in man. Br. J. Haematol. 1985, 61, 675–685.
- Stark, M.A.; Huo, Y.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of Apoptotic Neutrophils Regulates Granulopoiesis via IL-23 and IL-17. Immunity 2005, 22, 285–294.
- Martin, C.; Burdon, P.C.; Bridger, G.; Gutierrez-Ramos, J.-C.; Williams, T.J.; Rankin, S.M. Chemokines Acting via CXCR2 and CXCR4 Control the Release of Neutrophils from the Bone Marrow and Their Return following Senescence. Immunity 2003, 19, 583–593.
- Ley, K.; Hoffman, H.M.; Kubes, P.; Cassatella, M.A.; Zychlinsky, A.; Hedrick, C.C.; Catz, S.D. Neutrophils: New insights and open questions. Sci. Immunol. 2018, 3, eaat4579.
- Panopoulos, A.D.; Watowich, S.S. Granulocyte colony-stimulating factor: Molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 2008, 42, 277–288.
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358, 111–116.
- Arienti, S.; Barth, N.; Dorward, D.A.; Rossi, A.G.; Dransfield, I. Regulation of Apoptotic Cell Clearance During Resolution of Inflammation. Front. Pharmacol. 2019, 10, 891.
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898.
- Ortega-Gomez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674.
- Le Bousse-Kerdilès, M.; Martyré, M. Involvement of the fibrogenic cytokines, TGF-β and bFGF, in the pathogenesis of idiopathic myelofibrosis. Pathol. Biol. 2001, 49, 153–157.
- Luo, H.R.; Loison, F. Constitutive neutrophil apoptosis: Mechanisms and regulation. Am. J. Hematol. 2008, 83, 288–295.
- Grecian, R.; Whyte, M.K.B.; Walmsley, S. The role of neutrophils in cancer. Br. Med. Bull. 2018, 128, 5–14.
- Gray, R.D.; Hardisty, G.; Regan, K.H.; Smith, M.; Robb, C.T.; Duffin, R.; Mackellar, A.; Felton, J.M.; Paemka, L.; McCullagh, B.N.; et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax 2017, 73, 134–144.
- Fadok, V.A.; Bratton, D.L.; Guthrie, L.; Henson, P.M. Differential Effects of Apoptotic Versus Lysed Cells on Macrophage Production of Cytokines: Role of Proteases. J. Immunol. 2001, 166, 6847–6854.
This entry is offline, you can click here to edit this entry!
