Diabetic wounds are severe injuries that are common in patients that suffer from diabetes. Most of the presently employed wound dressing scaffolds are inappropriate for treating diabetic wounds. Improper treatment of diabetic wounds usually results in amputations. The shortcomings that are related to the currently used wound dressings include poor antimicrobial properties, inability to provide moisture, weak mechanical features, poor biodegradability, and biocompatibility, etc. To overcome the poor mechanical properties, polymer-based wound dressings have been designed from the combination of biopolymers (natural polymers) (e.g., chitosan, alginate, cellulose, chitin, gelatin, etc.) and synthetic polymers (e.g., poly (vinyl alcohol), poly (lactic-co-glycolic acid), polylactide, poly-glycolic acid, polyurethanes, etc.) to produce effective hybrid scaffolds for wound management.
- diabetic wounds
- polymers
- wound dressings
- bioactive agents
- and diabetic foot ulcer
1. Introduction
2. Polymer-Based Dressings Loaded with Bioactive Agents for Diabetic Wound Management
2.1. Nanofibers
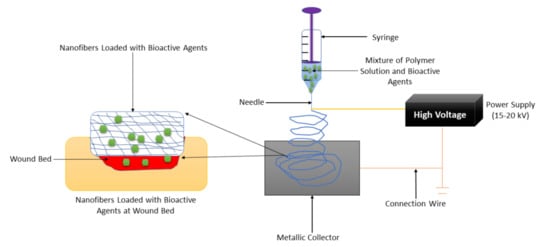
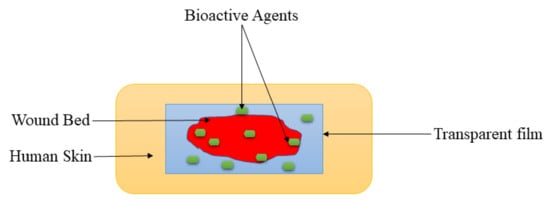
2.2. Films and Membranes
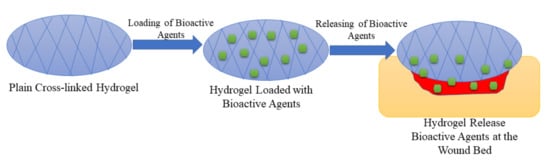
2.3. Hydrogels
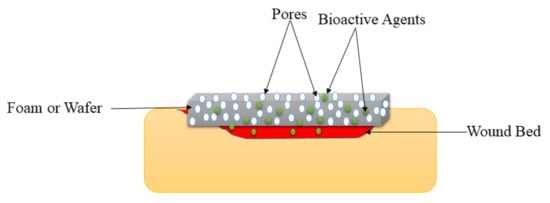
2.4. Foams and Wafers
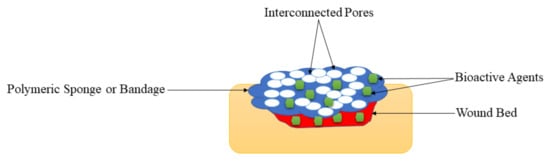
2.5. Sponges and Bandages
| Types of Wound Dressing | Used Polymers | Loaded Bioactive Agents | Results | Ref |
|---|---|---|---|---|
| Nanofiber | Gelatin and cellulose | Glybenclamide and metformin | Accelerated wound healing process and good biocompatibility | [57] |
| Nanofiber | PEG and PCL | EGF | Superior wound healing process | [58] |
| Nanofiber | Polylactide | Doxycycline | Excellent mechanical performance, antibacterial effects, and excellent diabetic wound healing properties | [59] |
| Nanofiber | PCL and gum tragacanth | Curcumin | Bead-free morphology and full wound closure on day 15. | [60] |
| Nanofiber | PU and carboxymethylcellulose | Malva sylvestris plant extract | Good diabetic wound healing rate | [61] |
| Nanofiber | Hydroxypropyl methylcellulose and PEO | beta-glucan | Non-toxic and accelerated wound closure. | [62] |
| Nanofiber | poly-N-acetyl glucosamine | polydeoxyribonucleotide | High rate of cell proliferation and angiogenesis. | [63] |
| Nanofiber | polyethersulfone | henceforth CD34+ cells | The fast diabetic wound healing process | [64] |
| Nanofiber | PCL | Bixin | Sustained drug release and accelerated wound healing. | [65] |
| Nanofiber | PLGA | PDGF, vancomycin, and gentamicin | Sustained drug release and accelerated wound healing. | [66] |
| Nanofiber | PCL | Sodium percarbonate | Superior vascularization. | [67] |
| Nanofiber | Cellulose acetate | Ag nanoparticles | High antibacterial efficacy and accelerated diabetic wound contraction. | [68] |
| Nanofiber | PCL | Curcumin | Excellent biocompatibility and increased rate of wound reduction | [69] |
| Nanofiber | PLGA | Insulin | Good mechanical performance and prolong drug release | [70] |
| Nanofibers | Chitosan and PVA | ZnO | Excellent antibacterial effects and accelerated diabetic wounds | [71] |
| Nanofibers | PVP and PCL | Pioglitazone | Non-toxicity and sustained drug release. | [72] |
| Film | Sodium alginate | Vicenin-2 | Faster diabetic wound recovery | [76] |
| Film | Chitosan | Alcoholic extracts | Excellent biocompatibility | [77] |
| Film | Chitosan | Fibroblast growth factors | High diabetic wound contraction rate | [78] |
| Film | Cellulose and PVA | Propolis and vitamin C | High swelling rate, controlled drug release, and accelerated diabetic wound healing | [79] |
| Film | Fibroin | Aloe gel | Excellent mechanical properties and fibroblast distribution and collagen fiber organization. | [80] |
| Film | Fibroin and chitosan | ADSCs | Good diabetic wound closure. | [81] |
| Film | PVA and cellulose | Curcumin | Good antibacterial effects and significantly diabetic wound closure. | [82] |
| Film | Chitosan | Retinoic acid | Increased wound reduction rate. | [83] |
| Film | Collagen | Biotinylated GHK peptide | Accelerated wound healing | [84] |
| Film | PVP and PVA | Sodium fusidate | Excellent mechanical performance | [85] |
| Film | Cellulose | Selenium | Fast diabetic wound healing rate | [86] |
| Membrane | PHBV | Cerium Oxide nanoparticles | Significant enhancement in cell infiltration and granulation tissue formation | [88] |
| Membrane | PVA and PLA | GFs | Excellent cell migration and proliferation | [89] |
| Membrane | HA | Human keratinocytes | The good clinical wound healing process | [90] |
| Membrane | PLGA and collagen | Glucophage | The faster wound healing process | [91] |
| Membrane | PLGA | Metformin | Enhanced the wound healing and re-epithelialization in diabetic rats | [92] |
| Membrane | PLLA | Dimethyloxalylglycine | Burst drug released followed by sustained drug release. | [93] |
| Membrane | Cellulose acetate | Sesamol | Improved diabetic wound healing | [94] |
| Membrane | PLGA and cellulose | Neurotensin | Sustained drug release and faster wound healing process | [95] |
| Hydrogel | Poly-ε-L-lysine, HA, and pluronic | Adipose mesenchymal stem cells | Increased diabetic wound rate | [100] |
| Hydrogel | HA and PEG | Stem cell | Good mechanical properties and faster diabetic wound healing. | [101] |
| Hydrogel | PEG and PVA | Fibroblasts and insulin | Accelerated wound repair | [102] |
| Hydrogel | HA | Human adipose stem cells | Improved wound closure rate | [103] |
| Hydrogel | Gelatin | Chemotactic cytokines | Accelerated wound healing | [104] |
| Hydrogel | Sodium carboxymethylcellulose | B. orientale | Fast wound recovery | [105] |
| Hydrogel | Pluronic F-127 | ADSCs | Accelerated wound healing | [106] |
| Hydrogel | PU | AASCs | Fast diabetic wound | [107] |
| Hydrogel | Chitosan | Exosomes | Accelerate angiogenesis and wound surface re-epithelialization | [108] |
| Hydrogel | PPCN | SDF-1 | Improved epithelial maturation and granulation tissue production | [109] |
| Hydrogel | Konjac glucomannan | Avena sativa | Support collagen expression, keratinocyte migration, fibroblast attachment, and proliferation | [110] |
| Hydrogel | Chitosan | L-glutamic acid | Promotes collagen deposition and accelerates vascularization | [111] |
| Hydrogel | Gelatin | Curcumin | Good cell migration | [112] |
| Hydrogel | Chitosan and PEG | Ag nanoparticles | Controlled drug release and diabetic wound stimulation. | [113] |
| Hydrogel | poly-(polyethyleneglycol citrate-co-N-isopropylacrylamide) | Copper metal-organic framework | Enhanced dermal cell migration and improved wound closure rates | [114] |
| Hydrogel | PVA | Nitric Oxide | Enhance diabetic wound healing | [115] |
| Hydrogel | HA | DNA | Enhanced development of granulation tissue | [116] |
| Hydrogel | poly (γ-glutamic acid) and chitosan | Superoxide dismutase | Good cytocompatibility and accelerated wound healing process | [117] |
| Hydrogel | Gelatin | Cerium-containing bioactive glass nanoparticles | Good antibacterial effects | [118] |
| Hydrogel | chitosan-dextran | Ag nanoparticles | Broad-spectrum and long-lasting antibacterial activity | [119] |
| Foam | PU | RhEGF | Moderate WVTR and good biocompatibility | [122] |
| Foam | PVA | Gentian violet and methylene blue | High wound reduction rate | [123] |
| Foam | PU | Ag nanoparticle | Fast wound healing rate | [124] |
| Foam | PU | Ag nanoparticle | Good antibacterial efficacy | [125] |
| Foam | PU | Ag | Good diabetic wound closure | [126] |
| Foam | Silk fibroin | Gastrodia elata and tea tree oil | High porosity and excellent biocompatibility | [127] |
| Foam | Chitosan | Neurotensin | High wound healing reduction | [128] |
| Foam | Silicone | Silver | Positive diabetic wound closure and reduction in size | [129] |
| Wafer | Calcium alginate | Ciprofloxacin | High porosity and burst drug release followed the sustained release with good antibacterial efficacy | [132] |
| Wafer | Xanthan gum | Silymarin | Good cell migration | [133] |
| Wafer | Sodium alginate and gelatin | Diosmin nanocrystals | Sustained drug release and well-developed granulation tissue, well-organized dermal layers, complete re-epithelialization, and mature collagen bundles in diabetic wounds. | [134] |
| Sponges | Chitosan and collagen | Recombinant human acidic fibroblast growth factors | Enhanced diabetic wound healing | [138] |
| Sponge | HA and chitosan | Ag nanoparticle | Good antibacterial effects and good cytocompatibility | [139] |
| Sponge | Chitosan | TMC nanoparticles | Faster diabetic wound healing | [140] |
| Sponge | Chitosan and HA | VEGFs | Burst release of GFs followed by sustained release. | [141] |
| Sponge | HA and collagen | EGF | Promoted blood vascular formation and granulation tissue development. | [142] |
| Sponge | Chitosan and silk | GMSC-derived exosomes | Enhanced deposition, re-epithelialization, and remodeling of ECM | [144] |
| Sponge | Collagen | Gementacin | Good pathogen eradication in diabetic wound | [143] |
| Sponge | Chitosan and alginate | Curcumin and honey | Sustained drug release and faster wound healing | [145] |
| Sponge | Chitosan and collagen | Thymosin beta 4 | Enhanced diabetic cutaneous wound healing | [146] |
| Bandages | Chitosan | ZnO nanoparticles | Good cytocompatibility and antibacterial effects. | [147] |
| Bandage | Chitin | ZnO nanoparticles | excellent antibacterial activity and high cell adhesion and migration | [149] |
| Bandage | Sodium alginate | EGF and curcumin | Non-toxicity and good biocompatibility | [148] |
| Bandage | Chitosan | Fluconazole and ciprofloxacin | High porosity and sustained drug release with good antimicrobial effects | [150] |
| Types of Wound Dressings | Advantages | Disadvantages | Highlights |
|---|---|---|---|
| Nanofibers | They possess a structure that mimics ECM, making them suitable for skin wound healing and regeneration. They are frequently formulated using efficient and easily employed electrospinning techniques. | It is not easy to produce nanofibers less than 10 nm in diameter. | The SEM micrographs of nanofibers loaded with bioactive agents display bead-free morphology that mimics ECM, making these wound dressings appropriate for providing an environment for cell proliferation and adhesion to accelerate the diabetic wound healing process. |
| Films and Membranes | These wound dressings are transparent, showing that the wound healing process can be observed without removing them. They also display good mechanical performance. | They are not suitable for exuding wounds due to their inability to absorb a large volume of biological fluids. | The mechanical properties of films and membranes were like those of human skin, making them skin compatible and easily handled during diabetic wound management. |
| Hydrogels | They are used as potential drug delivery systems in wound dressing applications and display other interesting properties such as high porosity, high swelling capacity, excellent biocompatibility, etc. | The biopolymer hydrogel dressings demonstrate poor mechanical performance that makes them not compatible with the human skin. | The drug release profiles were controlled release of bioactive agents from the polymeric hydrogels, resulting in an improved wound healing process. The high porosity of the hydrogels led to good swelling capability. |
| Foams and Wafers | These wound dressings exhibit high porosity that could provide cell growth and adhesion to accelerate the wound healing process. | They are not suitable for dry wounds. | The WVTR experiments of foams and wafers loaded with drugs exhibited moderate WTVR that can provide appropriate moisture to accelerate the healing of diabetic wounds. |
| Sponges and Bandages | These wound dressings are also displayed high porosity that could offer suitable gaseous permeation, superior cell proliferation, migration, and attachment for the accelerated wound healing process. | The very high porosity of the polymeric sponges or bandages can result in high uptake of wound exudate and high WVTR that may cause wound dehydration. | Polymeric sponges and bandages were mostly loaded with antibacterial agents for diabetic wound treatment, and they exhibited excellent antibacterial activity, demonstrating that these dressings are potential candidates for the management of infected diabetic wounds |
This entry is adapted from the peer-reviewed paper 10.3390/polym14040724
