The fabrication of porous Metal Organic Framework materials within resistant structures is a key challenge impeding their wide commercial use for processes such as adsorptive separation. In fact, the integration of nano-scale MOF crystallic structures into bulk components that can maintain the desired characteristics, i.e., size, shape, and mechanical stability, is a prerequisite for their wide practical use in many applications. At the same time, it requires sophisticated shaping techniques that can structure nano/micro-crystalline fine powders of MOFs into diverse types of macroscopic bodies such as monoliths. Under this framework, this review aims to bridge the gap between research advances and industrial necessities for fostering MOF applications into real life. Therefore, it critically explores recent advances in the shaping and production of MOF macro structures with regard to the binding materials that have received little attention to date, but have the potential to give new perspectives in the industrial applicability of MOFs.
- organic binders
- polymer binders
- inorganic binders
- metal–organic frameworks
- monoliths
- shape engineering
- applications
1. Introduction
2. Findings from Literature
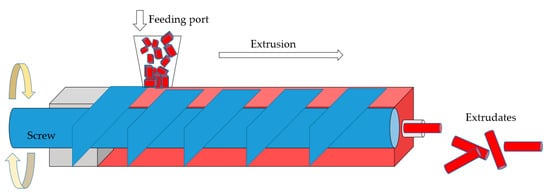
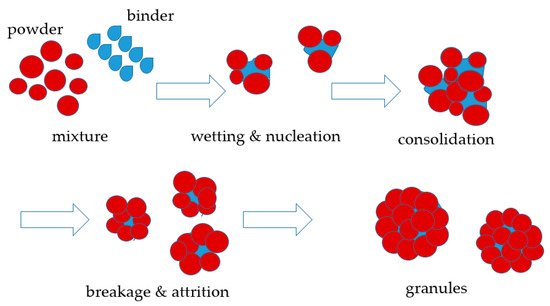
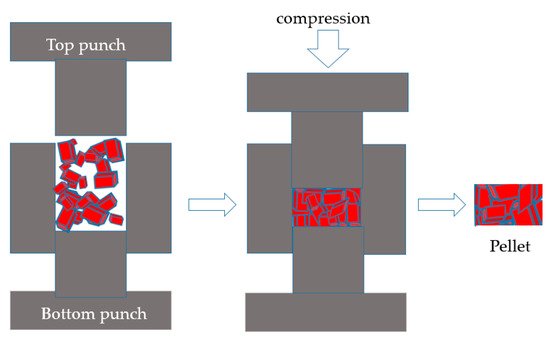
2.1. Processes with Organic Binders
|
MOF |
Binder (wt%) |
Shape |
SBET Powder (m2 g−1) |
SBET Body (m2 g−1) |
Application |
Reference |
|
TIFSIX-2-Cu-i |
PVB (10%) |
Pellet |
740 |
719 |
Gas separation and storage |
[27] |
|
SIFSIX-3-Ni |
PVB (10%) |
Pellet |
360 |
297 |
Gas separation and storage |
[27] |
|
GEFSIX-2-Cu-i |
PVB (10%) |
Pellet |
755 |
659 |
Gas separation and storage |
[27] |
|
SIFSIX-2-Cu-i |
PVB (9%) |
Pellet |
808 |
685 |
Gas separation and storage |
[27] |
|
ZIF-8 |
PEI (14%) |
Pellet |
- |
- |
Gas adsorption and separation |
[29] |
|
ZIF-8 |
PVC (23%) |
Pellet |
- |
- |
Gas adsorption and separation |
[29] |
|
ZIF-8 |
PVF (20%) |
Pellet |
- |
- |
Gas adsorption and separation |
[29] |
|
UiO66-COOH |
PVA (4%) |
Bead |
710 |
359 |
NH3 air purification |
[31] |
|
UiO66-COOH |
Polysiloxane (5.5%) |
Extrudate |
710 |
418 |
NH3 air purification |
[31] |
|
MOF-177 |
PVB (4%) |
Pellet |
2784 |
- |
CO2 adsorption |
[54] |
|
MOF-177-TEPA-20% |
PVB (4%) |
Pellet |
585 |
- |
CO2 adsorption |
[54] |
|
MOF-801 |
PVB (5%) |
Pellet |
899 |
569 |
CO2 and H2O adsorption |
[55] |
|
Zr-MOF |
Sucrose (10%) |
Pellet |
1367 |
674 |
H2 storage |
[56] |
|
UiO-66(Zr) |
PVA/PVB (3%) |
Granule |
1065 |
1017 |
Gas adsorption |
[57] |
|
UiO-66(Zr)_NH2 |
PVA/PVB (3%) |
Granule |
958 |
795 |
Gas adsorption |
[57] |
|
MIL-100(Fe) |
PVA/PVB (3%) |
Granule |
2261 |
2043 |
Gas adsorption |
[57] |
|
MIL-127(Fe) |
PVA/PVB (3%) |
Granule |
1181 |
1117 |
Gas adsorption |
[57] |
|
CPO-27(Ni) |
PVA (2%) |
Granule |
937 |
1319 |
NH3 adsorption for Respiratory protection filters |
[58] |
|
MIL-100(Fe) |
PVA (2%) |
Granule |
1212 |
1172 |
NH3 adsorption for Respiratory protection filters |
[58] |
|
Cu-BTC |
PVA (2%) |
Granule |
1605 |
147 |
NH3 adsorption for Respiratory protection filters |
[58] |
|
ZIF-8 |
PVF (15%) |
Bead |
- |
- |
Gas adsorption and separation |
[59] |
|
ZIF-8 |
PES (25%) |
Bead |
1384.4 |
1030.6 |
Oil sorption |
[60] |
|
Alfum |
PVA (20%) |
Monolith |
946 |
612 |
Water vapor sorption |
[61] |
|
MIL-160(Al) |
PVA (20%) |
Monolith |
1134 |
800 |
Water vapor sorption |
[61] |
|
MIL-101(Cr) |
PVA (20%) |
Monolith |
3171 |
2225 |
Water vapor sorption |
[61] |
|
Cu3(BTC)2 |
PVA (-) |
Pellet |
1737 |
963 |
H2O vapor and CO2 adsorption/catalysis |
[62] |
|
MIL-53(Al) |
PVA (13%) |
Pellet |
- |
- |
Separation of CO2/CH4 mixtures |
[63] |
|
UiO-66 |
PVA (1%) |
Monolith |
- |
- |
MOF shape engineering |
[64] |
|
UTSA-16 |
PVA (0–6.7%) |
Pellet |
- |
- |
CO2 adsorption |
[65] |
|
UTSA-16 |
PVA (-) |
Cylindrical extrudate |
- |
805 |
Gas adsorption (high pressure)/H2 purification from SMR off-gases |
[66] |
|
Alfum |
PVA (20%) |
Monolith |
1038 |
786 |
Water vapor sorption |
[67] |
|
MIL-101(Cr) |
PVA (20%) |
Monolith |
2731 |
1820 |
Water vapor sorption |
[67] |
|
UiO-66 |
PVA (-) |
Pellet |
1378 |
1274 |
CO2/N2 separation |
[68] |
|
MIL-101(Cr) |
PVA (10%) |
Grain |
2970 |
2610 |
Methanol adsorbent in adsorption heat transformation cycles |
[69] |
|
ZIF-8 |
PVA (2.9%) |
Cylindrical extrudate |
- |
- |
CO2/H2 Separation/biohydrogen purification |
[70] |
|
HKUST-1 |
PVA (2.9%) |
Cylindrical extrudate |
- |
- |
CO2/H2 Separation/biohydrogen purification |
[70] |
|
UTSA-16 |
PVA (2.9%) |
Cylindrical extrudate |
- |
- |
CO2/H2 Separation/biohydrogen purification |
[70] |
|
UiO-66 |
PVA (25%) |
Pellet |
1295 |
1031 |
Water adsorption heat transformation systems |
[71] |
|
Zr-fum |
PVA (25%) |
Pellet |
643 |
479 |
Water adsorption heat transformation systems |
[71] |
|
Al-fum |
PVA (25%) |
Pellet |
988 |
595 |
Water adsorption heat transformation systems |
[71] |
|
MIL-160 |
PVA (25%) |
Pellet |
1122 |
866 |
Water adsorption heat transformation systems |
[71] |
|
MIL-96(Al) |
PVA (0.5%) |
Monolith |
655 |
91 |
Upcycling of Li-ion batteries/MOF shape engineering |
[72] |
|
MOF-5 |
ABS (1/5/10%) |
3D-printed various geometries |
- |
- |
H2 adsorption/3D-printed H2 storage devices |
[73] |
|
ELM-11 |
PVP (10/20/31%) |
Pellet |
- |
- |
Gas storage and separation |
[74] |
|
MIL-100(Fe) |
PVP (2%) |
Monolith |
1917 |
1673.7 |
Ultra-low heat-driven atmospheric water harvesting (AWH) system |
[75] |
|
epn-MOF |
PVDF (30/40/50%) |
Bead |
- |
- |
CO2 capture in indoor environments |
[76] |
|
MIL-101(Cr) |
CMC sodium salt (5.5%) and starch (5.5%) |
Granule |
2471 |
1642 |
Gas (CO2, CH4, N2, CO) sorption |
[77] |
|
MIL-53(Al) |
MC 400 (10%) |
Cylindrical extrudate |
1525 |
1158 |
CO2 and CH4 adsorption |
[78] |
|
MIL-53(Al) |
MC 4000 (10%)) |
Cylindrical extrudate |
1525 |
1200 |
CO2 and CH4 adsorption |
[78] |
|
MIL-125(Ti)_NH2 |
Polyvinyl group (3%) |
Granule |
- |
- |
Syngas treatment aiming for H2 production |
[79] |
|
Mg-gallate |
HPC (4.8%) |
Pellet |
638 |
557 |
Hydrocarbon separations |
[80] |
|
Co-gallate |
HPC (4.8%) |
Pellet |
494 |
480 |
Hydrocarbon separations |
[80] |
|
Ni-gallate |
HPC (4.8%) |
Pellet |
455 |
425 |
Hydrocarbon separations |
[80] |
|
ZIF-8 |
HPC (0–40%) |
Granule |
- |
- |
MOF shape engineering |
[81] |
|
MIP-202 |
HPC (5%) |
Pellet |
278.6 |
- |
CO2/CH4 and CO2/N2 separation/CO2 capture |
[82] |
|
MIL-101(Cr). |
R,F-xerogel (50%) |
Monolith |
3060 |
1350 |
Water adsorption |
[83] |
|
MIL-100(Fe) |
R,F-xerogel (42%) |
Monolith |
2200 |
770 |
Water adsorption |
[83] |
|
MIL-100(Cr) |
R,F-xerogel (44%) |
Monolith |
1560 |
570 |
Water adsorption |
[83] |
|
ZIF-8 |
Cellulose ester (-) |
Tablet |
1433 |
1420 |
Catalysis/MOF shape engineering |
[84] |
|
HKUST-1 |
Cellulose ester (-) |
Tablet |
1897 |
453 |
Catalysis/MOF shape engineering |
[84] |
|
SIM-1 |
Cellulose ester (-) |
Tablet |
516 |
370 |
Catalysis/MOF shape engineering |
[84] |
2.2. Processes with Inorganic Binders
|
MOF |
Binder (wt%) |
Shape |
SBET Powder (m2 g−1) |
SBET Body (m2 g−1) |
Application |
Reference |
|
MIL-100(Fe) |
ρ-alumina (5%) |
Sphere |
2088 |
1831 |
Ammonia adsorption |
[30] |
|
MIL-101(Cr) |
ρ-alumina (5%) |
Sphere |
4066 |
3685 |
CO2 adsorption |
[30] |
|
UIO-66(Zr) |
ρ-alumina (5%) |
Sphere |
1050 |
911 |
CO2 adsorption |
[30] |
|
UIO-66_NH2 |
ρ-alumina (5%) |
Sphere |
875 |
823 |
CO2 adsorption |
[30] |
|
ZIF-8 |
Bentonite clay (10%) |
Tablet |
1022.8 |
820.6 |
- |
[89] |
|
ZIF-8 |
Alumina (10%) |
Tablet |
1022.8 |
947.9 |
- |
[89] |
|
ZIF-8 |
SB powder (10%) |
Tablet |
1022.8 |
959.2 |
Gas adsorption |
[89] |
|
ZIF-8 |
Talc powder (10%) |
Tablet |
1022.8 |
951.3 |
Gas adsorption |
[89] |
|
ZIF-8 |
Sesbania powder (10%) |
Tablet |
1022.8 |
846.4 |
- |
[89] |
|
ZIF-8 |
Silica (10%) |
Tablet |
1022.8 |
945.9 |
- |
[89] |
|
UiO-66 |
Graphite (1%) |
Tablet |
1140 |
885 |
Selective adsorption and separation of xylene isomers |
[90] |
|
ZIF-8 |
Bentonite (20%) |
Monolith |
1415 |
1083 |
Adsorptive Separations/biobutanol recovery |
[91] |
|
ZIF-8 |
Bentonite (16.7%) and MC (16.7%) |
Monolith |
1415 |
1070 |
Adsorptive Separations/biobutanol recovery |
[91] |
|
HKUST-1 |
Bentonite (15%) and MC (15%) |
Pellet |
1271.2 |
605.1 |
MOF shape engineering |
[92] |
|
ZIF-8 |
Bentonite (15%) and MC (15%) |
Pellet |
2047 |
1471.5 |
MOF shape engineering |
[92] |
|
ZIF-67 |
Bentonite (15%) and MC (15%) |
Pellet |
1789.6 |
464.4 |
MOF shape engineering |
[92] |
|
UiO-66 |
Bentonite (15%) and MC (15%) |
Pellet |
1110.8 |
187.4 |
MOF shape engineering |
[92] |
|
MIL-101(Cr) |
Bentonite (25%) |
Monolith |
- |
- |
CO2 adsorption |
[93] |
|
MIL-101 (Cr) |
Bentonite (25/40%) |
Monolith |
- |
- |
CO2 adsorption |
[94] |
|
MIL-100(Fe) |
Silica sol (10%) |
Granule |
1772 |
1619 |
Separation of SF6 from SF6/N2 mixture |
[95] |
|
UiO-66(Zr) |
ρ-alumina (5%) |
Bead |
903 |
619 |
Room temperature gas adsorption/H2O and CH4 adsorption |
[96] |
|
MIL-100(Fe) |
ρ-alumina (5%) |
Bead |
1928 |
1451 |
Room temperature gas adsorption/H2O and CH4 adsorption |
[96] |
|
MIL-127(Fe) |
ρ-alumina (5%) |
Bead |
1413 |
1266 |
Room temperature gas adsorption/H2O and CH4 adsorption |
[96] |
|
MIL-101 |
Sodium silicate and starch (7%) |
Pellet |
2730 |
1910 |
CO2 adsorption |
[97] |
|
MIL-100(Fe) |
Silica (10%) |
Granule |
- |
1568 |
C2/C3 hydrocarbon separation |
[98] |
|
Cu3(BTC)2 |
Silres MSE 100 (13.8%) |
Monolith |
- |
484 |
MOF shape engineering |
[99] |
|
ZIF-8 |
ρ-alumina (5/10/15%) |
Pellet |
- |
- |
CH4/N2 separation |
[100] |
|
MIL-53(Al) |
ρ-alumina (5/10/15%) |
Pellet |
- |
- |
CH4/N2 separation |
[100] |
|
ZIF-7 |
Silica (15%) |
Monolith |
16 |
40 |
Adsorption of ethane and ethylene |
[101] |
|
MOF-74(Ni) |
Bentonite (15%) and PVA (5%) |
Monolith |
1180 |
737 |
Gas adsorption |
[102] |
|
UTSA-16(Co) |
Bentonite (10%) and PVA (5%) |
Monolith |
727 |
568 |
Gas adsorption |
[102] |
|
MIL-101 |
Bentonite (15%) and PVA (5%) |
Monolith |
2400 |
2200 |
CO2 removal from enclosed environments |
[103] |
3.3. Processes without Binders
|
MOF |
Binder |
Shape |
SBET Powder (m2 g−1) |
SBET Body (m2 g−1) |
Application |
Reference |
|
Cu3(BTC)2 |
None |
Monolith |
307 |
834 |
No application/MOF shape engineering |
[28] |
|
UiO-66 |
None |
Tablet |
1426 |
1459 |
No application/MOF shape engineering |
[104] |
|
UiO-67 |
None |
Tablet |
2034 |
1549 |
No application/MOF shape engineering |
[104] |
|
UiO-66-NH2 |
None |
Tablet |
839 |
625 |
No application/MOF shape engineering |
[104] |
|
HKUST-1 |
None |
Tablet |
1288 |
1091 |
No application/MOF shape engineering |
[104] |
|
ZIF-8HT |
None |
Monolith |
- |
1387 |
No application/MOF shape engineering |
[105] |
|
ZIF-8LT |
None |
Monolith |
- |
1359 |
No application/MOF shape engineering |
[105] |
|
ZIF-8LT-HT |
None |
Monolith |
- |
1423 |
No application/MOF shape engineering |
[105] |
|
ZIF-8ER |
None |
Monolith |
- |
1395 |
No application/MOF shape engineering |
[105] |
|
HKUST-1 |
None |
Monolith |
- |
1193 |
Methane adsorption |
[106] |
|
UiO-66 |
None |
Sphere |
1167 |
1127 |
No application/MOF shape engineering |
[108] |
|
MOF-5 |
None |
Pellet |
2762 |
2707 |
Hydrogen storage |
[109] |
|
CPO-27-Ni |
None |
Pellet |
- |
- |
Methane storage |
[110] |
|
CuBTC |
None |
Pellet |
- |
- |
CO2 capture |
[111] |
|
MIL-53(Al) |
None |
Pellet |
- |
- |
CO2 capture |
[111] |
|
HKUST-1 |
None |
monolith |
- |
1134 |
Methane storage |
[112] |
|
HKUST-1 |
None |
tablet |
1850 |
- |
Methane storage/MOF shape engineering |
[113] |
This entry is adapted from the peer-reviewed paper 10.3390/en15041489
References
- Sriram, G.; Bendre, A.; Mariappan, E.; Altalhi, T.; Kigga, M.; Ching, Y.C.; Jung, H.-Y.; Bhaduri, B.; Kurkuri, M. Recent trends in the application of metal-organic frameworks (MOFs) for the removal of toxic dyes and their removal mechanism-a review. Sustain. Mater. Technol. 2022, 31, e00378.
- Ntouros, V.; Kousis, I.; Papadaki, D.; Pisello, A.L.; Assimakopoulos, M.N. Life Cycle Assessment on Different Synthetic Routes of ZIF-8 Nanomaterials. Energies 2021, 14, 4998.
- Papaefstathiou, G.S.; Friščić, T.; MacGillivray, L.R. Design and Construction of a 2D Metal Organic Framework with Multiple Cavities: A Nonregular Net with a Paracyclophane that Codes for Multiply Fused Nodes. J. Am. Chem. Soc. 2005, 127, 14160–14161.
- Polyakov, V.A.; Butova, V.V.; Erofeeva, E.A.; Tereshchenko, A.A.; Soldatov, A.V. MW Synthesis of ZIF-7. The Effect of Solvent on Particle Size and Hydrogen Sorption Properties. Energies 2020, 13, 6306.
- Samanidou, V.F.; Deliyanni, E.A. Metal Organic Frameworks: Synthesis and Application. Molecules 2020, 25, 960.
- Al Obeidli, A.; Ben Salah, H.; Al Murisi, M.; Sabouni, R. Recent advancements in MOFs synthesis and their green applications. Int. J. Hydrogen Energy 2022, 47, 2561–2593.
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal–organic frameworks. Coord. Chem. Rev. 2021, 426, 213544.
- Valizadeh, B.; Nguyen, T.N.; Smit, B.; Stylianou, K.C. Porous Metal–Organic Beads for Iodine Capture and Recovery Using a Gas-Sparged Column. Adv. Funct. Mater. 2018, 28, 1801596.
- Al-Rowaili, F.N.; Zahid, U.; Onaizi, S.; Khaled, M.; Jamal, A.; AL-Mutairi, E.M. A review for Metal-Organic Frameworks (MOFs) utilization in capture and conversion of carbon dioxide into valuable products. J. CO2 Util. 2021, 53, 101715.
- Hou, J.; Sapnik, A.F.; Bennett, T.D. Metal–organic framework gels and monoliths. Chem. Sci. 2020, 11, 310–323.
- Stanley, P.M.; Warnan, J. Molecular Dye-Sensitized Photocatalysis with Metal-Organic Framework and Metal Oxide Colloids for Fuel Production. Energies 2021, 14, 4260.
- Samantaray, S.S.; Putnam, S.T.; Stadie, N.P. Volumetrics of Hydrogen Storage by Physical Adsorption. Inorganics 2021, 9, 45.
- Yeskendir, B.; Dacquin, J.-P.; Lorgouilloux, Y.; Courtois, C.; Royer, S.; Dhainaut, J. From metal–organic framework powders to shaped solids: Recent developments and challenges. Mater. Adv. 2021, 2, 7139–7186.
- Shah, B.B.; Kundu, T.; Zhao, D. Mechanical Properties of Shaped Metal-Organic Frameworks. Top. Curr. Chem. Cham 2019, 377, 25.
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape engineering of metal–organic frameworks. Polyhedron 2018, 145, 1–15.
- Lorignon, F.; Gossard, A.; Carboni, M. Hierarchically porous monolithic MOFs: An ongoing challenge for industrial-scale effluent treatment. Chem. Eng. J. 2020, 393, 124765.
- Aguilera-Sigalat, J.; Bradshaw, D. A colloidal water-stable MOF as a broad-range fluorescent pH sensor via post-synthetic modification. Chem. Commun. 2014, 50, 4711–4713.
- Wu, J.; Dai, Q.; Zhang, H.; Li, X. A defect-free MOF composite membrane prepared via in-situ binder-controlled restrained second-growth method for energy storage device. Energy Storage Mater. 2021, 35, 687–694.
- Zheng, J.; Cui, X.; Yang, Q.; Ren, Q.; Yang, Y.; Xing, H. Shaping of ultrahigh-loading MOF pellet with a strongly anti-tearing binder for gas separation and storage. Chem. Eng. J. 2018, 354, 1075–1082.
- Zhang, B.; Zhang, J.; Liu, C.; Peng, L.; Sang, X.; Han, B.; Ma, X.; Luo, T.; Tan, X.; Yang, G. High-internal-phase emulsions stabilized by metal-organic frameworks and derivation of ultralight metal-organic aerogels. Sci. Rep. 2016, 6, 21401.
- Cousin-Saint-Remi, J.; Finoulst, A.-L.; Jabbour, C.; Baron, G.V.; Denayer, J.F.M. Selection of binder recipes for the formulation of MOFs into resistant pellets for molecular separations by fixed-bed adsorption. Microporous Mesoporous Mater. 2020, 304, 109322.
- Valekar, A.H.; Cho, K.-H.; Lee, U.-H.; Lee, J.S.; Yoon, J.W.; Hwang, Y.K.; Lee, S.G.; Cho, S.J.; Chang, J.-S. Shaping of porous metal–organic framework granules using mesoporous ρ-alumina as a binder. RSC Adv. 2017, 7, 55767–55777.
- Khabzina, Y.; Dhainaut, J.; Ahlhelm, M.; Richter, H.-J.; Reinsch, H.; Stock, N.; Farrusseng, D. Synthesis and Shaping Scale-up Study of Functionalized UiO-66 MOF for Ammonia Air Purification Filters. Ind. Eng. Chem. Res. 2018, 57, 8200–8208.
- Lee, U.-H.; Valekar, A.H.; Hwang, Y.K.; Chang, J.-S. Granulation and Shaping of Metal–Organic Frameworks. In The Chemistry of Metal–Organic Frameworks, 1st ed.; Kaskel, S., Ed.; Wiley-VCH: Weinheim, Germany, 2016; pp. 551–572.
- Liu, D.; Purewal, J.J.; Yang, J.; Sudik, A.; Maurer, S.; Mueller, U.; Ni, J.; Siegel, D.J. MOF-5 composites exhibiting improved thermal conductivity. Int. J. Hydrogen Energy 2012, 37, 6109–6117.
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279.
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444.
- Ogawa, T.; Iyoki, K.; Fukushima, T.; Kajikawa, Y. Landscape of Research Areas for Zeolites and Metal-Organic Frameworks Using Computational Classification Based on Citation Networks. Materials 2017, 10, 1428.
- Govender, S.; Friedrich, H.B. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catalysts 2017, 7, 62.
- Free, Z.; Hernandez, M.; Mashal, M.; Mondal, K. A Review on Advanced Manufacturing for Hydrogen Storage Applications. Energies 2021, 14, 8513.
- Mahmoud, E.; Ali, L.; El Sayah, A.; Alkhatib, S.A.; Abdulsalam, H.; Juma, M.; Al-Muhtaseb, A.H. Implementing Metal-Organic Frameworks for Natural Gas Storage. Crystals 2019, 9, 406.
- Connolly, B.M.; Madden, D.G.; Wheatley, A.E.H.; Fairen-Jimenez, D. Shaping the Future of Fuel: Monolithic Metal–Organic Frameworks for High-Density Gas Storage. J. Am. Chem. Soc. 2020, 142, 8541–8549.
- Kalaj, M.; Bentz, K.C.; Ayala, S.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures. Chem. Rev. 2020, 120, 8267–8302.
- Boussemghoune, M.; Chikhi, M.; Ozay, Y.; Guler, P.; Ozbey Unal, B.; Dizge, N. The Investigation of Organic Binder Effect on Morphological Structure of Ceramic Membrane Support. Symmetry 2020, 12, 770.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 11199, Polyvinyl Alcohol. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11199 (accessed on 11 February 2022).
- Olabisi, O.; Adewale, K. (Eds.) Handbook of Thermoplastics, 2nd ed.; Plastics Engineering; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-4665-7722-0.
- Ida, S. PES (Poly(ether sulfone)), Polysulfone. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–8. ISBN 978-3-642-36199-9.
- Fluorinated Coatings and Finishes Handbook-2nd Edition. Available online: https://www.elsevier.com/books/fluorinated-coatings-and-finishes-handbook/mckeen/978-0-323-37126-1 (accessed on 13 February 2022).
- Poly(vinylpyrrolidone). CAS Common Chemistry. CAS, a Division of the American Chemical Society, n.d. Available online: https://commonchemistry.cas.org/detail?cas_rn=9003-39-8 (accessed on 13 February 2022).
- Methyl Cellulose|9004-67-5. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3474718.htm (accessed on 13 February 2022).
- Hydroxypropyl Cellulose|9004-64-2. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB0241870.htm (accessed on 13 February 2022).
- Gaikwad, S.; Kim, Y.; Gaikwad, R.; Han, S. Enhanced CO2 capture capacity of amine-functionalized MOF-177 metal organic framework. J. Environ. Chem. Eng. 2021, 9, 105523.
- Taddei, M.; McPherson, M.J.; Gougsa, A.; Lam, J.; Sewell, J.; Andreoli, E. An Optimised Compaction Process for Zr-Fumarate (MOF-801). Inorganics 2019, 7, 110.
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Swartbooi, A.; North, B.C.; Mathe, M. A more efficient way to shape metal-organic framework (MOF) powder materials for hydrogen storage applications. Int. J. Hydrogen Energy 2015, 40, 4617–4622.
- Chanut, N.; Wiersum, A.D.; Lee, U.-H.; Hwang, Y.K.; Ragon, F.; Chevreau, H.; Bourrelly, S.; Kuchta, B.; Chang, J.-S.; Serre, C.; et al. Observing the Effects of Shaping on Gas Adsorption in Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2016, 2016, 4416–4423.
- Hindocha, S.; Poulston, S. Study of the scale-up, formulation, ageing and ammonia adsorption capacity of MIL-100(Fe), Cu-BTC and CPO-27(Ni) for use in respiratory protection filters. Faraday Discuss. 2017, 201, 113–125.
- Cousin-Saint-Remi, J.; Van der Perre, S.; Segato, T.; Delplancke, M.-P.; Goderis, S.; Terryn, H.; Baron, G.; Denayer, J. Highly Robust MOF Polymeric Beads with a Controllable Size for Molecular Separations. ACS Appl. Mater. Interfaces 2019, 11, 13694–13703.
- Abbasi, Z.; Shamsaei, E.; Fang, X.-Y.; Ladewig, B.; Wang, H. Simple fabrication of zeolitic imidazolate framework ZIF-8/polymer composite beads by phase inversion method for efficient oil sorption. J. Colloid Interface Sci. 2017, 493, 150–161.
- Hastürk, E.; Höfert, S.-P.; Topalli, B.; Schlüsener, C.; Janiak, C. Shaping of MOFs via freeze-casting method with hydrophilic polymers and their effect on textural properties. Microporous Mesoporous Mater. 2020, 295, 109907.
- Kim, J.; Kim, S.-H.; Yang, S.-T.; Ahn, W.-S. Bench-scale preparation of Cu3(BTC)2 by ethanol reflux: Synthesis optimization and adsorption/catalytic applications. Microporous Mesoporous Mater. 2012, 161, 48–55.
- Finsy, V.; Ma, L.; Alaerts, L.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Separation of CO2/CH4 mixtures with the MIL-53(Al) metal–organic framework. Microporous Mesoporous Mater. 2009, 120, 221–227.
- Zhu, H.; Zhang, Q.; Zhu, S. Assembly of a Metal–Organic Framework into 3 D Hierarchical Porous Monoliths Using a Pickering High Internal Phase Emulsion Template. Chem.–Eur. J. 2016, 22, 8751–8755.
- Grande, C.A.; Águeda, V.I.; Spjelkavik, A.; Blom, R. An efficient recipe for formulation of metal-organic Frameworks. Chem. Eng. Sci. 2015, 124, 154–158.
- Agueda, V.I.; Delgado, J.A.; Uguina, M.A.; Brea, P.; Spjelkavik, A.I.; Blom, R.; Grande, C. Adsorption and diffusion of H2, N2, CO, CH4 and CO2 in UTSA-16 metal-organic framework extrudates. Chem. Eng. Sci. 2015, 124, 159–169.
- Hastürk, E.; Schlüsener, C.; Quodbach, J.; Schmitz, A.; Janiak, C. Shaping of metal-organic frameworks into mechanically stable monoliths with poly(vinyl alcohol) by phase separation technique. Microporous Mesoporous Mater. 2019, 280, 277–287.
- Edubilli, S.; Gumma, S. A systematic evaluation of UiO-66 metal organic framework for CO2/N2 separation. Sep. Purif. Technol. 2019, 224, 85–94.
- Solovyeva, M.V.; Gordeeva, L.G.; Aristov, Y.I. “MIL-101(Cr)–methanol” as working pair for adsorption heat transformation cycles: Adsorbent shaping, adsorption equilibrium and dynamics. Energy Convers. Manag. 2019, 182, 299–306.
- Delgado, J.A.; Águeda, V.I.; Uguina, M.A.; Brea, P.; Grande, C.A. Comparison and evaluation of agglomerated MOFs in biohydrogen purification by means of pressure swing adsorption (PSA). Chem. Eng. J. 2017, 326, 117–129.
- Gökpinar, S.; Ernst, S.-J.; Hastürk, E.; Möllers, M.; El Aita, I.; Wiedey, R.; Tannert, N.; Nießing, S.; Abdpour, S.; Schmitz, A.; et al. Air-Con Metal–Organic Frameworks in Binder Composites for Water Adsorption Heat Transformation Systems. Ind. Eng. Chem. Res. 2019, 58, 21493–21503.
- Lorignon, F.; Gossard, A.; Carboni, M.; Meyer, D. From wastes to interconnected porous monolith: Upcycling of Al-based metal organic framework via pickering emulsion template. Mater. Lett. 2021, 296, 129931.
- Kreider, M.C.; Sefa, M.; Fedchak, J.A.; Scherschligt, J.; Bible, M.; Natarajan, B.; Klimov, N.N.; Miller, A.E.; Ahmed, Z.; Hartings, M.R. Toward 3D printed hydrogen storage materials made with ABS-MOF composites. Polym. Adv. Technol. 2018, 29, 867–873.
- Hiraide, S.; Arima, H.; Tanaka, H.; Miyahara, M.T. Slacking of Gate Adsorption Behavior on Metal–Organic Frameworks under an External Force. ACS Appl. Mater. Interfaces 2021, 13, 30213–30223.
- Maher, H.; Rupam, T.H.; Rocky, K.A.; Bassiouny, R.; Saha, B.B. Silica gel-MIL 100(Fe) composite adsorbents for ultra-low heat-driven atmospheric water harvester. Energy 2022, 238, 121741.
- Park, J.; Chae, Y.S.; Kang, D.W.; Kang, M.; Choe, J.H.; Kim, S.; Kim, J.Y.; Jeong, Y.W.; Hong, C.S. Shaping of a Metal–Organic Framework–Polymer Composite and Its CO2 Adsorption Performances from Humid Indoor Air. ACS Appl. Mater. Interfaces 2021, 13, 25421–25427.
- Munusamy, K.; Sethia, G.; Patil, D.V.; Somayajulu Rallapalli, P.B.; Somani, R.S.; Bajaj, H.C. Sorption of carbon dioxide, methane, nitrogen and carbon monoxide on MIL-101(Cr): Volumetric measurements and dynamic adsorption studies. Chem. Eng. J. 2012, 195–196, 359–368.
- Kriesten, M.; Vargas Schmitz, J.; Siegel, J.; Smith, C.E.; Kaspereit, M.; Hartmann, M. Shaping of Flexible Metal-Organic Frameworks: Combining Macroscopic Stability and Framework Flexibility. Eur. J. Inorg. Chem. 2019, 2019, 4700–4709.
- Regufe, M.J.; Tamajon, J.; Ribeiro, A.M.; Ferreira, A.; Lee, U.-H.; Hwang, Y.K.; Chang, J.-S.; Serre, C.; Loureiro, J.M.; Rodrigues, A.E. Syngas Purification by Porous Amino-Functionalized Titanium Terephthalate MIL-125. Energy Fuels 2015, 29, 4654–4664.
- Wu, K.; Guo, L.; Zhang, Z.; Yang, Q.; Yang, Y.; Ren, Q.; Bao, Z. Shaping of gallate-based metal-organic frameworks for adsorption separation of ethylene from acetylene and ethane. J. Colloid Interface Sci. 2021, 581, 177–184.
- Ohsaki, S.; Nakahara, Y.; Nakamura, H.; Watano, S. Flowability improvement of soft metal-organic framework particles by wet granulation. Microporous Mesoporous Mater. 2020, 293, 109785.
- Lv, D.; Chen, J.; Yang, K.; Wu, H.; Chen, Y.; Duan, C.; Wu, Y.; Xiao, J.; Xi, H.; Li, Z.; et al. Ultrahigh CO2/CH4 and CO2/N2 adsorption selectivities on a cost-effectively L-aspartic acid based metal-organic framework. Chem. Eng. J. 2019, 375, 122074.
- Wickenheisser, M.; Herbst, A.; Tannert, R.; Milow, B.; Janiak, C. Hierarchical MOF-xerogel monolith composites from embedding MIL-100(Fe,Cr) and MIL-101(Cr) in resorcinol-formaldehyde xerogels for water adsorption applications. Microporous Mesoporous Mater. 2015, 215, 143–153.
- Bazer-Bachi, D.; Assié, L.; Lecocq, V.; Harbuzaru, B.; Falk, V. Towards industrial use of metal-organic framework: Impact of shaping on the MOF properties. Powder Technol. 2014, 255, 52–59.
- ICSC 0384-BENTONITE. Available online: https://inchem.org/documents/icsc/icsc/eics0384.htm (accessed on 14 February 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 24261, Silicon Dioxide. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/24261 (accessed on 14 February 2022).
- Xie, Y.; Kocaefe, D.; Kocaefe, Y.; Cheng, J.; Liu, W. The Effect of Novel Synthetic Methods and Parameters Control on Morphology of Nano-alumina Particles. Nanoscale Res. Lett. 2016, 11, 259.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9989226, Aluminum Oxide. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9989226 (accessed on 14 February 2022).
- Zhu, J.; Jiang, L.; Dai, C.; Yang, N.; Lei, Z. Gas adsorption in shaped zeolitic imidazolate framework-8. Chin. J. Chem. Eng. 2015, 23, 1275–1282.
- Moreira, M.A.; Santos, J.C.; Ferreira, A.F.P.; Loureiro, J.M.; Ragon, F.; Horcajada, P.; Shim, K.-E.; Hwang, Y.-K.; Lee, U.-H.; Chang, J.-S.; et al. Reverse Shape Selectivity in the Liquid-Phase Adsorption of Xylene Isomers in Zirconium Terephthalate MOF UiO-66. Langmuir 2012, 28, 5715–5723.
- Lefevere, J.; Claessens, B.; Mullens, S.; Baron, G.; Cousin-Saint-Remi, J.; Denayer, J.F.M. 3D-Printed Zeolitic Imidazolate Framework Structures for Adsorptive Separations. ACS Appl. Nano Mater. 2019, 2, 4991–4999.
- Tsalaporta, E.; MacElroy, J.M.D. A comparative study of the physical and chemical properties of pelletized HKUST-1, ZIF-8, ZIF-67 and UiO-66 powders. Heliyon 2020, 6, e04883.
- Hong, W.Y.; Perera, S.P.; Burrows, A.D. Comparison of MIL-101(Cr) metal-organic framework and 13X zeolite monoliths for CO2 capture. Microporous Mesoporous Mater. 2020, 308, 110525.
- Hong, W.Y.; Perera, S.P.; Burrows, A.D. Manufacturing of metal-organic framework monoliths and their application in CO2 adsorption. Microporous Mesoporous Mater. 2015, 214, 149–155.
- Kim, P.-J.; You, Y.-W.; Park, H.; Chang, J.-S.; Bae, Y.-S.; Lee, C.-H.; Suh, J.-K. Separation of SF6 from SF6/N2 mixture using metal–organic framework MIL-100(Fe) granule. Chem. Eng. J. 2015, 262, 683–690.
- Iacomi, P.; Lee, U.-H.; Valekar, A.H.; Chang, J.-S.; Llewellyn, P.L. Investigating the effect of alumina shaping on the sorption properties of promising metal–organic frameworks. RSC Adv. 2019, 9, 7128–7135.
- Montazerolghaem, M.; Aghamiri, S.F.; Talaie, M.R.; Tangestaninejad, S. A comparative investigation of CO2 adsorption on powder and pellet forms of MIL-101. J. Taiwan Inst. Chem. Eng. 2017, 72, 45–52.
- Martins, V.F.D.; Seabra, R.; Silva, P.; Ribeiro, A.M.; Cho, K.H.; Lee, U.-H.; Chang, J.-S.; Loureiro, J.M.; Rodrigues, A.E.; Ferreira, A. C2/C3 Hydrocarbon Separation by Pressure Swing Adsorption on MIL-100(Fe). Ind. Eng. Chem. Res. 2020, 59, 10568–10582.
- Küsgens, P.; Zgaverdea, A.; Fritz, H.-G.; Siegle, S.; Kaskel, S. Metal-Organic Frameworks in Monolithic Structures. J. Am. Ceram. Soc. 2010, 93, 2476–2479.
- Pereira, A.; Ferreira, A.F.P.; Rodrigues, A.; Ribeiro, A.M.; Regufe, M.J. Shaping of ZIF-8 and MIL-53(Al) adsorbents for CH4/N2 separation. Microporous Mesoporous Mater. 2022, 331, 111648.
- Thakkar, H.; Al-Naddaf, Q.; Legion, N.; Hovis, M.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Adsorption of Ethane and Ethylene over 3D-Printed Ethane-Selective Monoliths. ACS Sustain. Chem. Eng. 2018, 6, 15228–15237.
- Thakkar, H.; Eastman, S.; Al-Naddaf, Q.; Rownaghi, A.A.; Rezaei, F. 3D-Printed Metal–Organic Framework Monoliths for Gas Adsorption Processes. ACS Appl. Mater. Interfaces 2017, 9, 35908–35916.
- Lawson, S.; Griffin, C.; Rapp, K.; Rownaghi, A.A.; Rezaei, F. Amine-Functionalized MIL-101 Monoliths for CO2 Removal from Enclosed Environments. Energy Fuels 2019, 33, 2399–2407.
- Dhainaut, J.; Avci-Camur, C.; Troyano, J.; Legrand, A.; Canivet, J.; Imaz, I.; Maspoch, D.; Reinsch, H.; Farrusseng, D. Systematic study of the impact of MOF densification into tablets on textural and mechanical properties. CrystEngComm 2017, 19, 4211–4218.
- Tian, T.; Velazquez-Garcia, J.; Bennett, T.D.; Fairen-Jimenez, D. Mechanically and chemically robust ZIF-8 monoliths with high volumetric adsorption capacity. J. Mater. Chem. A 2015, 3, 2999–3005.
- Tian, T.; Zeng, Z.; Vulpe, D.; Casco, M.E.; Divitini, G.; Midgley, P.A.; Silvestre-Albero, J.; Tan, J.-C.; Moghadam, P.Z.; Fairen-Jimenez, D. A sol–gel monolithic metal–organic framework with enhanced methane uptake. Nat. Mater. 2018, 17, 174–179.
- Gallagher, J. Towards methane targets. Nat. Energy 2018, 3, 86.
- Bueken, B.; Velthoven, N.V.; Willhammar, T.; Stassin, T.; Stassen, I.; Keen, D.A.; Baron, G.V.; Denayer, J.F.M.; Ameloot, R.; Bals, S.; et al. Gel-based morphological design of zirconium metal–organic frameworks. Chem. Sci. 2017, 8, 3939–3948.
- Purewal, J.J.; Liu, D.; Yang, J.; Sudik, A.; Siegel, D.J.; Maurer, S.; Müller, U. Increased volumetric hydrogen uptake of MOF-5 by powder densification. Int. J. Hydrogen Energy 2012, 37, 2723–2727.
- Tagliabue, M.; Rizzo, C.; Millini, R.; Dietzel, P.D.C.; Blom, R.; Zanardi, S. Methane storage on CPO-27-Ni pellets. J. Porous Mater. 2011, 18, 289–296.
- Majchrzak-Kucęba, I.; Ściubidło, A. Shaping metal–organic framework (MOF) powder materials for CO2 capture applications—A thermogravimetric study. J. Therm. Anal. Calorim. 2019, 138, 4139–4144.
- Lim, G.J.H.; Wu, Y.; Shah, B.B.; Koh, J.J.; Liu, C.K.; Zhao, D.; Cheetham, A.K.; Wang, J.; Ding, J. 3D-Printing of Pure Metal–Organic Framework Monoliths. ACS Mater. Lett. 2019, 1, 147–153.
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane Storage in Metal–Organic Frameworks: Current Records, Surprise Findings, and Challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894.
