Although stem cells have been considered promising for the treatment of degenerative diseases by ‘seeding’ them into damaged tissues, it has recently been observed that the regenerative capacity of stem cells is influenced and regulated by the local immune response and in particular by macrophages, which constitute a central component of the damage response and are the coordinators of tissue repair and regeneration. Among the panoply of immune cells involved in the response to both acute and chronic wounds, recent discoveries have highlighted novel and often unexpected roles for certain types of immune cells in promoting a permissive local environment for effective cell replacement and restoration of tissue integrity. Some studies have shown that the control of inflammation is crucial in regenerative therapies: To be effective, regenerative therapies must block and control inflammation to allow tissue regeneration by resident stem cells. Indeed, the presence of inflammation inhibits the regenerative action of tissue-resident mesenchymal stem cells (MSCs). Recent papers suggest that an innovative regenerative strategy could be to polarize macrophages from the M1 inflammatory state to the M2 anti-inflammatory state utilizing immune cells. These reviews conclude that next-generation regenerative therapies need an immune-centric approach instead of the use of stem cells. Thus, depending on the tissue or organ targeted, regenerative strategies could be developed to stimulate macrophage polarization or to recruit subpopulations of pro-healing macrophages. Already, Mordechai in 2013 and Pinto in 2014 have shown that the regeneration of myocardial tissue after ischemia was induced by macrophages that regulate resident stem cells and promote regeneration, suggesting that targeting macrophages could be a new strategy to improve infarct healing and repair. The regenerative and stem-cell-controlling capacity of macrophages has also recently been demonstrated in bone tissue by Gibon et al., Gullard et al., and Ekstrom. Najar et al. in 2018 clarified that mesenchymal stem cells act through a paracrine and immune-modulatory and non-differentiative mechanism and that the microenvironment and immune system regulate the activity of MSCs regardless of the tissue from which they originate. Based on the role played by several types of macrophages and lymphocytes in the wound-healing response, it is tempting to hypothesize that interventions that reduce the M1 macrophage phenotype and promote M2 may represent a new therapy to induce tissue regeneration.
- wound healing
- diabetic foot
- immune system
- monocytes
- lymphocytes
- immune centric revolution
1. How to Switch to M2 Regenerative Phenotype?
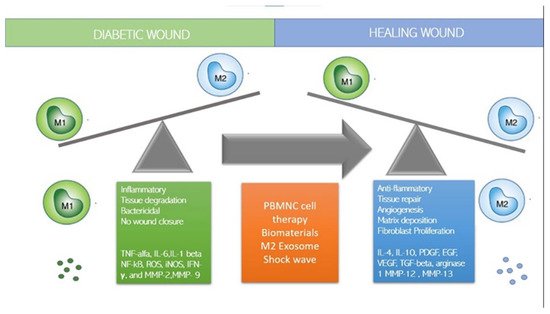
2. Immune-Cell-Based Cell Therapy
3. Mesenchymal Stem Cells (MSC)
4. Extracellular Vesicles (EVs) and Exosome (Exo)
5. Dermal Substitutes
This entry is adapted from the peer-reviewed paper 10.3390/jcm11030889
References
- Zuloff-Shani, A.; Kachel, E.; Frenkel, O.; Orenstein, A.; Shinar, E.; Danon, D. Macrophage suspensions prepared from a blood unit for treatment of refractory human ulcers. Transfus. Apher. Sci. 2004, 30, 163–167.
- Danon, D.; Madjar, J.; Edinov, E.; Knyszynski, A.; Brill, S.; Diamantshtein, L.; Shinar, E. Treatment of human ulcers by application of macrophages prepared from a blood unit. Exp. Gerontol. 1997, 32, 633–641.
- Zuloff-Shani, A.; Adunsky, A.; Even-Zahav, A.; Semo, H.; Orenstein, A.; Tamir, J.; Regev, E.; Shinar, E.; Danon, D. Hard to heal pressure ulcers (stage III–IV): Efficacy of injected activated macrophage suspension (AMS) as compared with standard of care (SOC) treatment controlled trial. Arch. Gerontol. Geriatr. 2010, 51, 268–272.
- Magenta, A.; Florio, M.C.; Ruggeri, M.; Furgiuele, S. Autologous cell therapy in diabetes-associated critical limb ischemia: From basic studies to clinical outcomes (Review). Int. J. Mol. Med. 2020, 48, 173.
- Moriya, J.; Minamino, T.; Tateno, K.; Shimizu, N.; Kuwabara, Y.; Sato, Y.; Saito, Y.; Komuro, I. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ. Cardiovasc. Interv. 2009, 2, 245–254.
- Huang, P.P.; Yang, X.F.; Li, S.Z.; Wen, J.C.; Zhang, Y.; Han, Z.C. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb. Haemost. 2007, 98, 1335–1342.
- Liotta, F.; Annunziato, F.; Castellani, S.; Boddi, M.; Alterini, B.; Castellini, G.; Mazzanti, B.; Cosmi, L.; Acquafresca, M.; Bartalesi, F.; et al. Therapeutic Efficacy of Autologous Non-Mobilized Enriched Circulating Endothelial Progenitors in Patients with Critical Limb Ischemia―The SCELTA Trial. Circ. J. 2018, 82, 1688–1698.
- Dubsky, M.; Jirkovska, A.; Bem, R.; Fejfarova, V.; Pagacova, L.; Sixta, B.; Varga, M.; Langkramer, S.; Sykova, E.; Jude, E.B. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab. Res. Rev. 2013, 29, 369–376.
- Persiani, F.; Paolini, A.; Camilli, D.; Mascellari, L.; Platone, A.; Magenta, A.; Furgiuele, S. Peripheral Blood Mononuclear Cells Therapy for Treatment of Lower Limb Ischemia in Diabetic Patients: A Single-Center Experience. Ann. Vasc. Surg. 2018, 53, 190–196.
- De Angelis, B.; Gentile, P.; Orlandi, F.; Bocchini, I.; Di Pasquali, C.; Agovino, A.; Gizzi, C.; Patrizi, F.; Scioli, M.G.; Orlandi, A.; et al. Limb Rescue: A New Autologous-Peripheral Blood Mononuclear Cells Technology in Critical Limb Ischemia and Chronic Ulcers. Tissue Eng. Part C Methods 2015, 21, 423–435.
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ. Res. 2017, 120, 1326–1340.
- Liew, A.; Bhattacharya, V.; Shaw, J.; Stansby, G. Cell Therapy for Critical Limb Ischemia: A Meta-Analysis of Randomized Controlled Trials. Angiology 2016, 67, 444–455.
- Zhang, Y.; Deng, H.; Tang, Z. Efficacy of Cellular Therapy for Diabetic Foot Ulcer: A Meta-Analysis of Randomized Controlled Clinical Trials. Cell Transplant. 2018, 26, 1931–1939.
- Guo, J.; Dardik, A.; Fang, K.; Huang, R.; Gu, Y. Meta-analysis on the treatment of diabetic foot ulcers with autologous stem cells. Stem Cell Res. Ther. 2017, 8, 228.
- Shu, X.; Shu, S.; Tang, S.; Yang, L.; Liu, D.; Li, K.; Dong, Z.; Ma, Z.; Zhu, Z.; Din, J. Efficiency of stem cell based therapy in the treatment of diabetic foot ulcer: A meta-analysis. Endocr. J. 2018, 65, 403–413.
- Dubský, M.; Jirkovská, A.; Bem, R.; Fejfarová, V.; Pagacová, L.; Nemcová, A.; Sixta, B.; Chlupac, J.; Peregrin, J.H.; Syková, E.; et al. Comparison of the effect of stem cell therapy and percutaneous transluminal angioplasty on diabetic foot disease in patients with critical limb ischemia. Cytotherapy 2014, 16, 1733–1738.
- Spaltro, G.; Straino, S.; Gambini, E.; Bassetti, B.; Persico, L.; Zoli, S.; Zanobini, M.; Capogrossi, M.C.; Spirito, R.; Quarti, C.; et al. Characterization of the Pall Celeris system as a point-of-care device for therapeutic angiogenesis. Cytotherapy 2015, 17, 1302–1313.
- Scatena, A.; Petruzzi, P.; Maioli, F.; Lucaroni, F.; Ventoruzzo, G.; Bolognese, L.; Ercolini, L. Does Autologous Peripheral Mononuclear Cells Implant Allow Foot Surgery in Diabetic Patients with Critical Limb Ischaemia not Eligible for Revascularization? In Proceedings of the 8th International Symposium Diabetic Foot, IDF, The Hague, The Netherlands, 22–25 May 2019; p. 95.
- Di Vieste, G.; Formenti, I.; Balduzzi, G.; Masserini, B.; De Giglio, R. Use of autologous peripheral blood mononuclear cells in a case of diabetic foot. J. AMD 2019, 22, 230–233.
- Di Pardo, A.; Cappello, E.; Pepe, G.; Marracino, F.; Carrieri, V.; Maglione, V.; Pompeo, F. Infusion of autologous-peripheral blood mononuclear cells: A new approach for limb salvage in patients with diabetes. In Proceedings of the 7th International Diabetic Foot Congress Abu Dhabi, IFD Congress, Abu Dhabi, United Arab Emirates, 4–8 December 2017.
- Jetten, N.; Roumans, N.; Gijbels, M.J.; Romano, A.; Post, M.J.; de Winther, M.P.J.; Van Der Hulst, R.R.W.J.; Xanthoulea, S. Wound Administration of M2-Polarized Macrophages Does Not Improve Murine Cutaneous Healing Responses. PLoS ONE 2014, 9, e102994.
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.J.; Donners, M.M.P.C. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118.
- Saldaña, L.; Bensiamar, F.; Vallés, G.; Mancebo, F.J.; García-Rey, E.; Vilaboa, N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res. Ther. 2019, 10, 58.
- Anton, K.; Banerjee, D.; Glod, J. Macrophage-Associated Mesenchymal Stem Cells Assume an Activated, Migratory, Pro-Inflammatory Phenotype with Increased IL-6 and CXCL10 Secretion. PLoS ONE 2012, 7, e35036.
- Saldaña, L.; Vallés, G.; Bensiamar, F.; Mancebo, F.J.; García-Rey, E.; Vilaboa, N. Paracrine interactions between mesenchymal stem cells and macrophages are regulated by 1,25-dihydroxyvitamin D3. Sci. Rep. 2017, 7, 14618.
- Harrell, C.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467.
- Harrell, C.R.; Djonov, V.; Volarevic, V. The cross-talk between mesenchymal stem cells and immune cells in tissue repair and regeneration. Int. J. Mol. Sci. 2021, 22, 2472.
- Zhou, Y.; Singh, A.K.; Hoyt, R.F.; Wang, S.; Yu, Z.; Hunt, T.; Kindzelski, B.; Corcoran, P.C.; Mohiuddin, M.M.; Horvath, K.A. Regulatory T cells enhance mesenchymal stem cell survival and proliferation following autologous cotransplantation in ischemic myocardium. J. Thorac. Cardiovasc. Surg. 2014, 148, 1131–1137.
- Faulknor, R.A.; Olekson, M.A.; Ekwueme, E.C.; Krzyszczyk, P.; Freeman, J.W.; Berthiaume, F. Hypoxia impairs mesenchymal stromal cell-induced macrophage M1 to M2 transition. Technology 2017, 5, 81–86.
- Inoue, O.; Usui, S.; Takashima, S.; Nomura, A.; Yamaguchi, K.; Takeda, Y.; Goten, C.; Hamaoka, T.; Ootsuji, H.; Murai, H.; et al. Diabetes impairs the angiogenic capacity of human adipose-derived stem cells by reducing the CD271+ subpopulation in adipose tissue. Biochem. Biophys. Res. Commun. 2019, 517, 369–375.
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553.
- Spiller, K.L.; Koh, T.J. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliv. Rev. 2017, 122, 74–83.
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Chasing the recipe for a pro-regenerative immune system. Semin. Cell Dev. Biol. 2017, 61, 71–79.
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev. 2018, 14, 337–345.
- Fadini, G.P.; Spinetti, G.; Santopaolo, M.; Madeddu, P. Impaired Regeneration Contributes to Poor Outcomes in Diabetic Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 34–44.
- Santopaolo, M.; Sambataro, M.; Spinetti, G.; Madeddu, P. Bone marrow as a target and accomplice of vascular complications in diabetes. Diabetes Metab. Res. Rev. 2020, 36, e3240.
- Wang, J.; Xia, J.; Huang, R.; Hu, Y.; Fan, J.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles alter disease outcomes via endorsement of macrophage polarization. Stem Cell Res. Ther. 2020, 11, 424.
- Heo, J.S.; Choi, Y.; Kim, H.O.; Matta, C. Adipose-Derived Mesenchymal Stem Cells Promote M2 Macrophage Phenotype through Exosomes. Stem Cells Int. 2019, 2019, 7921760.
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B.; Bolontrade, M.F. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708.
- Li, M.; Wang, T.; Tian, H.; Wei, G.; Zhao, L.; Shi, Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3793–3803.
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. 2019, 6, 1900513.
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 29.
- Varela, P.; Sartori, S.; Viebahn, R.; Salber, J.; Ciardelli, G. Macrophage immunomodulation: An indispensable tool to evaluate the performance of wound dressing biomaterials. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019830355.
- Whiterel, C.E.; Graney, P.L.; Freytes, D.O.; Weingarten, M.S.; Spiller, K.L. Response of human macrophages to wound matrices in vitro. Wound Repair Regen. 2016, 24, 514–524.
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655.
- Tylek, T.; Blum, C.; Hrynevich, A.; Schlegelmilch, K.; Schilling, T.; Dalton, P.D.; Groll, J. Precisely defined fiber scaffolds with 40 μm porosity induce elongation driven M2-like polarization of human macrophages. Biofabrication 2020, 12, 025007.
- Yin, Y.; He, X.T.; Wang, J.; Wu, R.X.; Xu, X.Y.; Hong, Y.L.; Tian, B.M.; Chen, F.M. Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl. Mater. Today 2020, 18, 100466.
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Liu, Z.; Huang, H. Immune-enhancing activities of chondroitin sulfate in murine macrophage RAW 264.7 cells. Carbohydr. Polym. 2018, 198, 611–619.
- Agrawal, H.; Tholpady, S.S.; Capito, A.E.; Drake, D.B.; Katz, A.J. Macrophage phenotypes correspond with remodeling outcomes of various acellular dermal matrices. Open J. Regen. Med. 2012, 1, 51–59.
- Paige, J.T.; Kremer, M.; Landry, J.; Hatfield, S.A.; Wathieu, D.; Brug, A.; Lightell, D.J.; Spiller, K.L.; Woods, T.C. Modulation of inflammation in wounds of diabetic patients treated with porcine urinary bladder matrix. Regen. Med. 2019, 14, 269–277.
- Montanaro, M.; Meloni, M.; Anemona, L.; Servadei, F.; Smirnov, A.; Candi, E. Macrophage Activation and M2 Polarization in Wound Bed of Diabetic Patients Treated by Dermal/Epidermal Substitute Nevelia. Int. J. Low. Extrem. Wounds, 2020; in press.
- Uccioli, L.; Meloni, M.; Izzo, V.; Giurato, L. Use of Nevelia Dermal-Epidermal Regenerative Template in the Management of Ischemic Diabetic Foot Postsurgical Wounds. Int. J. Low. Extrem. Wounds 2020, 19, 282–288.
- Swartzwelter, B.J.; Verde, A.; Rehak, L.; Madej, M.; Puntes, V.F.; De Luca, A.C.; Boraschi, D.; Italiani, P. Interaction between macrophages and nanoparticles: In vitro 3d cultures for the realistic assessment of inflammatory activation and modulation of innate memory. Nanomaterials 2021, 11, 207.
