We review the progress in metal phosphate structural chemistry focused on proton conductivity properties and applications. Attention is paid to structure–property relationships, which ultimately determine the potential use of metal phosphates and derivatives in devices relying on proton conduction. The origin of their conducting properties, including both intrinsic and extrinsic conductivity, is rationalized in terms of distinctive structural features and the presence of specific proton carriers or the factors involved in the formation of extended hydrogen-bond networks. To make the exposition of this large class of proton conductor materials more comprehensive, we group/combine metal phosphates by their metal oxidation state, starting with metal (IV) phosphates and pyrophosphates, considering historical rationales and taking into account the accumulated body of knowledge of these compounds. We highlight the main characteristics of super protonic CsH2PO4, its applicability, as well as the affordance of its composite derivatives. We finish by discussing relevant structure–conducting property correlations for divalent and trivalent metal phosphates. Overall, emphasis is placed on materials exhibiting outstanding properties for applications as electrolyte components or single electrolytes in Polymer Electrolyte Membrane Fuel Cells and Intermediate Temperature Fuel Cells.
- metal phosphate
- proton conductivity
- H-bond network
- proton carriers
- super protonic
- metal pyrophosphate
1. Introduction
Metal phosphates (MPs) comprise an ample class of structurally versatile acidic solids, with outstanding performances in a wide variety of applications, such as catalysts [1][2][3], fuel cells [4][5][6], batteries [7], biomedical [8], etc. Depending on the metal/phosphate combinations and the synthetic methodologies, MP solids can be prepared in a vast diversity of crystalline forms, from 3D open-frameworks, through layered networks, to 1D polymeric structures.
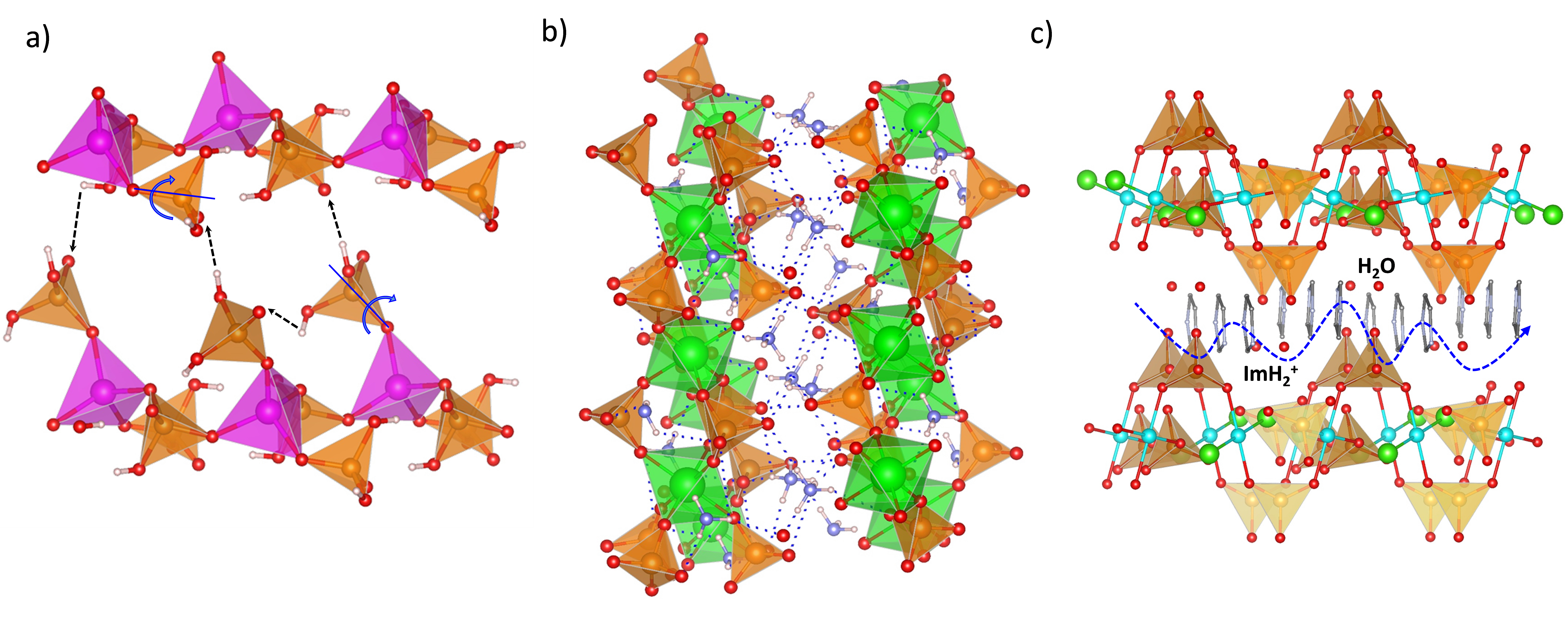
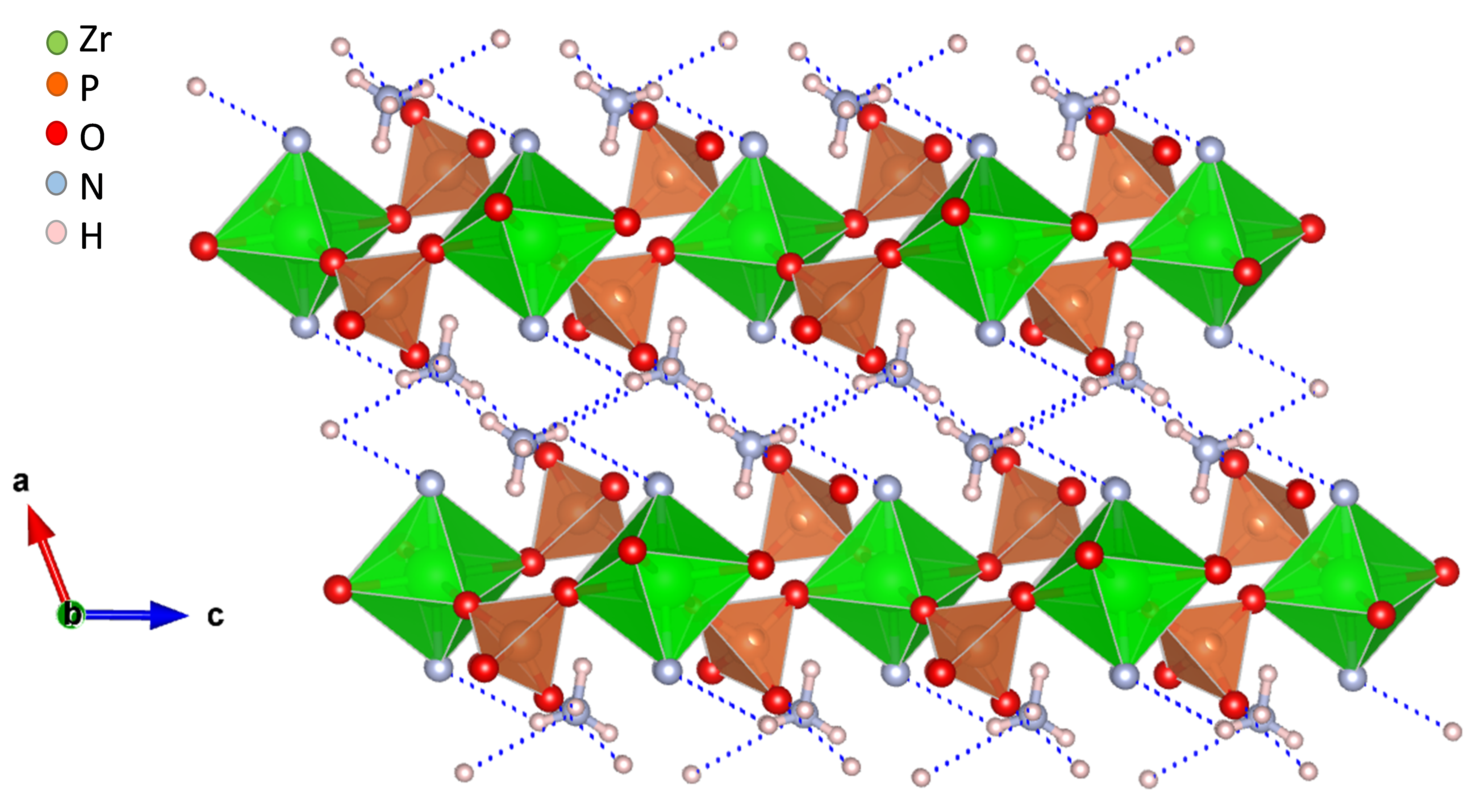
2. Tetravalent Metal Phosphates and Pyrophosphates
2.1. Zirconium Phosphates

2.2. Zirconium Phosphate Composite Membranes
2.3. Titanium and Tin(IV) Phosphates
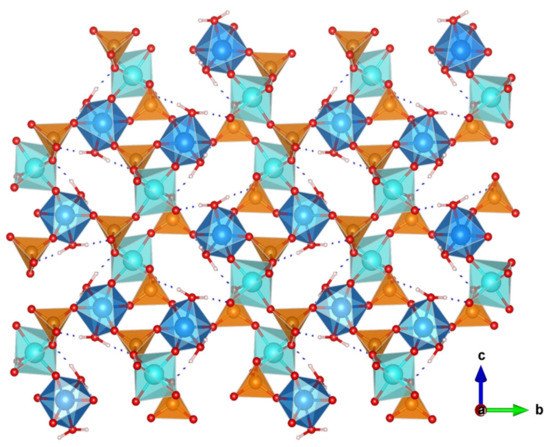
2.4. Other Tetravalent Phosphates
2.5. Tetravalent Pyrophosphates
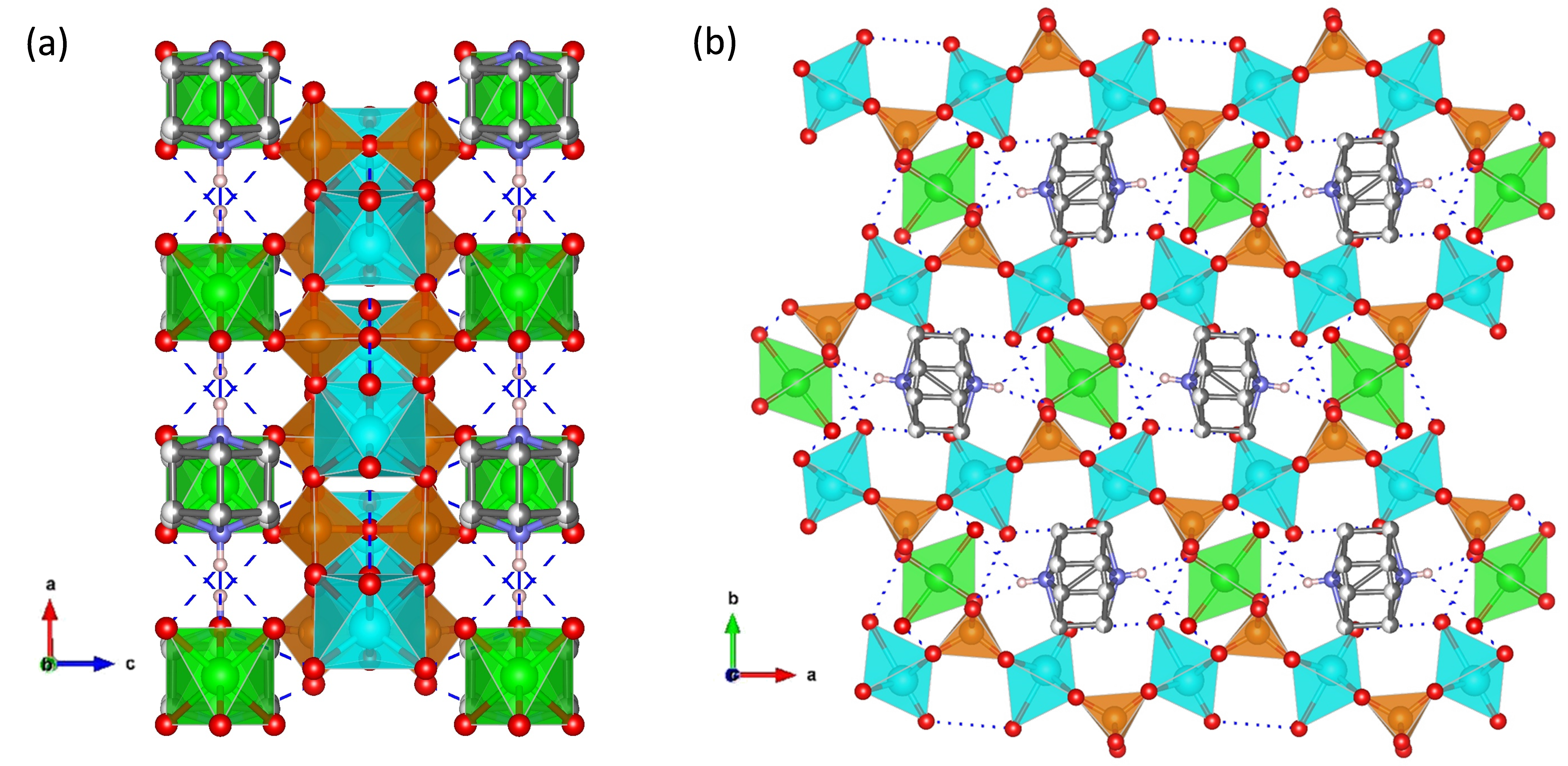
3. Super Protonic Metal(I) Phosphates
Solid acid proton conductors, with stoichiometry MIHyXO4 (MI = Cs, Rb; X = S, P, Se; y = 1, 2), have received much attention because they exhibit exceptional proton transport properties and can be used as electrolytes in fuel cells operated at intermediate temperatures (120-300 °C). The fundamental characteristics of these materials are the phase transition that occurs in response to heating, cooling or application of pressure [6][119], accompanied by an increase in proton conductivity of several orders of magnitude—referred to as super protonic conductivity (Figure 7).
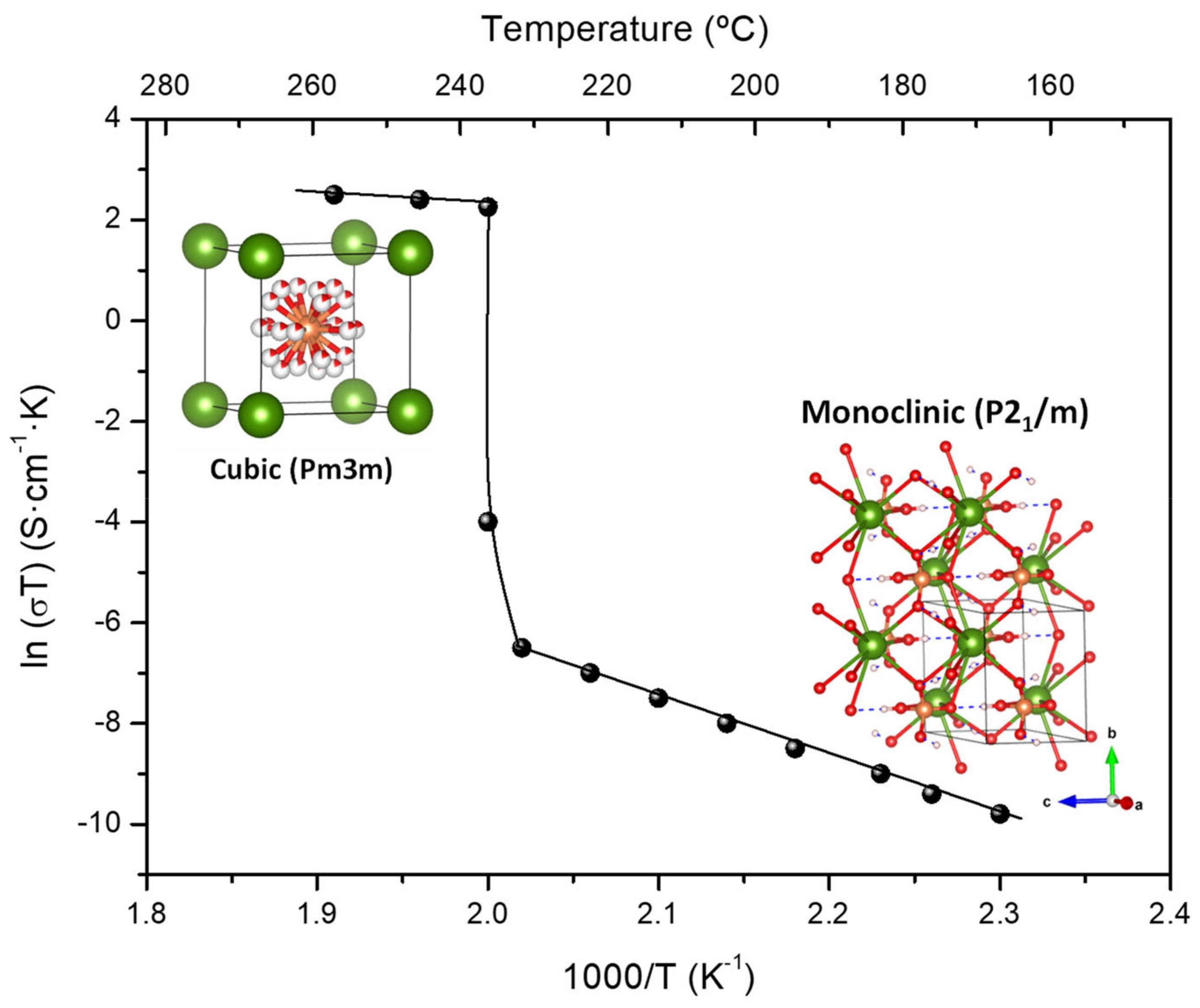
Figure 7. The characteristic high and low temperature proton conductivity and the corresponding structures for CsH2PO4, adapted from [6][120].
This property has been associated with the delocalization of hydrogen bonds [120]. For CsH2PO4, a proton conductivity of 6 × 10-2 S·cm-1 (Table 1) was measured above 230 °C corresponding to the super protonic cubic (Pm-3m) phase [121] while it drastically drops in the low temperature phases. Recently, from an ab initio molecular dynamics simulation study of the solid acids CsHSeO4, CsHSO4 and CsH2PO4, it was concluded that efficient long-range proton transfer in the high temperature (HT) phases is enabled by the interplay of high proton-transfer rates and frequent anion reorientation.
In these compounds, proton conduction follows a Grotthuss mechanism with proton transfer being associated with structural reorientation [122]. The super protonic conductor CsH2PO4 is stable under humidified conditions (PH2O = 0.4 atm) [6], but it dehydrates to CsPO3, via the transient phase Cs2H2P2O7, at 230-260 °C, according to the relationship log(PH2O/atm) = 6.11(±0.82) - 3.63(±0.42) × 1000/(Tdehy/K) [123].desired product [6].
Improvements in the mechanical and proton conductivity properties, as well as in thermal stability of CsH2PO4-based electrolytes have been addressed by mixing with oxide materials, such as zirconia, silica, alumina, and titania (Table 1). Thus, the (1-x)Cs3(HSO4)2(H2PO4)/xSiO2 composite with x = 0.7 increase the proton conductivity up to 10–2 S·cm−1 in the range 60–200 °C. Moreover, the introduction of fine-particle silica reduced the jump in conductivity at the phase-transition temperature and shifted it to lower temperatures. Higher silica contents led to a decrease in the conductivity due to the disruption of conduction paths [132].
The use of acid-modified silica confers high thermal stability at low H2O partial pressure while maintaining high proton conductivity (10−3–10−2 S·cm−1, at 130–250 °C) [134]. A similar trend was found for the composites (1−x)CsH2PO4/xTiO2 and (1−x)CsH2PO4/xZrO2 (contents x = 0.1 and 0.2) [135][136]. High performance was also reported for the composite 8:1:1 CsH2PO4/NaH2PO4/ZrO2, with a stable conductivity of 2.23 × 10−2 S·cm−1 for 42 h being measured for the high temperature phase [137]. Nanodiamonds (ND) are another type of heterogeneous additive giving rise to the (1−x)CsH2PO4-xND (x = 0–0.5) composites exhibiting enhanced low temperature proton conductivities, while maintaining almost unaltered that of high temperature [140].
CsH2PO4 composites based on organic additives have been also intensively investigated. For example, binary mixtures containing N-heterocycles (1,2,4-triazole, benzimidazole and imidazole) displayed enhanced proton conductivity at temperatures below the super protonic phase transition (2 − 8 × 10−4 S·cm−1 at 174–190 °C) [138]. Another approach was reported in which the solid acid CsH2PO4 was combined with fluoroelastomer p(VDF/HFP), producing high conductive composite membranes (1−x)CsH2PO4-xp(VDF/HPF) (x = 0.05–0.25 wt%) with improved mechanical and hydrophobic properties, along with flexibility and reduced thickness.
However, a high concentration of p(VDF/HFP) rendered membranes with a reduced proton conductivity for the HT phase [141]. By using the polymer butvar (polyvinyl butyral) [121], composite membranes (1−x)CsH2PO4-xButvar (x < 0.2 wt%) were obtained showing, in a wide range of composition, a proton conduction behaviour analogous to the pure salt in the high temperature region but with increased low temperature conductivity by three orders of magnitude at x = 0.2.
Table 1. Proton conductivity data for selected monovalent and tetravalent metal phosphates and pyrophosphates.
|
Compounds/Dimensionality |
Temperature (°C)/RH(%) |
Conductivity (S·cm-1) |
Ea (eV) |
Ref. |
|
Tetravalent Metal Phosphates |
|
|
|
|
|
ZrP·0.8PrNH2·5H2O/2D |
20/90 |
1.2 × 10-3 |
1.04 |
[50] |
|
Zr(P2O7)0.81(O3POH)0.38/2D |
20/90 |
1.3 × 10-3 |
0.19 |
[51] |
|
Zr(O3POH)0.65(O3PC6H4SO3H)1.35/2D |
100/90 |
7.0 × 10-2 |
--- |
[52] |
|
(NH4)2[ZrF2(HPO4)2]/3D |
90/95 |
1.45 × 10-2 |
0.19 |
[53] |
|
(NH4)5[Zr3(OH)3F6(PO4)2(HPO4)]/3D |
60/98 |
4.41 × 10-2 |
0.33 |
[54] |
|
(NH4)3Zr(H2/3PO4)3/1D |
90/95 |
1.21 × 10-2 |
0.30 |
[55] |
|
Ti2(HPO4)4/1D |
20/95 |
1.2 × 10-3 |
0.13 |
[83] |
|
Ti2O(PO4)2·2H2O (π-TiP)/3D |
90/95 |
1.3 × 10-3 |
0.23 |
[86] |
|
Ti(HPO4)1(O3PC6H4SO3H)0.85(OH)0.30·nH2O/2D |
100/-- |
0.1 |
0.18 |
[117] |
|
Sn(HPO4)2·3H2O/2D |
100/95 |
1.0 × 10-2 |
--- |
[88] |
|
α-ZrP2O7/3D |
300 |
1.0 × 10−4 |
--- |
[49] |
|
(NH4)3Zr(H2/3PO4)3/1D |
180 |
1.45 × 10−3 |
0.26 |
[55] |
|
Tetravalent Pyrophosphates |
|
|
|
|
|
TiP2O7/3D |
100/100 |
4.4 × 10-3 |
0.14 |
[116] |
|
(C6H14N2)[NiV2O6H8(P2O7)2]·2H2O/3D |
60/100 |
2.0 × 10-2 |
0.38 |
[118] |
|
In0.1Sn0.9P2O7/3D |
300 |
0.195 |
--- |
[108] |
|
Ce0.9Mg0.1P2O7/3D |
200 |
4.0 × 10−2 |
--- |
[115] |
|
CeP2O7/3D |
180 |
3.0 × 10−2 |
--- |
[115] |
|
Super protonic Cesium Phosphates |
|
|
|
|
|
CsH2PO4/3D |
>230 |
6.0 × 10−2 |
--- |
[120] |
|
Cs1-xRbxH2PO4/3D |
240 |
3.0 × 10−2 |
0.92 |
[127] |
|
Cs1−xH2+xPO4/3D |
150 |
2.0 × 10−2 |
0.70 |
[141] |
|
(1−x)Cs3(HSO4)2(HPO4)/xSiO2 |
200 |
1.0 × 10−2 |
--- |
[132] |
|
(1-x)CsH2PO4/xTiO2 |
230 |
2.0 × 10−2 |
--- |
[135] |
|
(1-x)CsH2PO4/xZrO2 |
250 |
2.6 × 10−2 |
--- |
[136] |
|
CsH2PO4/NaH2PO4/ZrO2 |
230 |
2.23 × 10−2 |
--- |
[137] |
![]() 4. Divalent and Trivalent Metal Phosphates
4. Divalent and Trivalent Metal Phosphates
4.1. Divalent Metal Phosphates
Divalent transition metal phosphates show a great structural versatility, from 1D polymeric topologies through layered framework to 3D open-framework structures. Most of these solids are synthetized in the presence of organic molecules, which are retained as protonated guest species (amines, iminazole derivatives, etc.), thus, compensating the anionic charge of the inorganic framework. This is formed by the metal ion, mainly in octahedral or tetrahedral coordination environments, linked to the phosphate groups with different protonated degrees (HxPO4). The presence of the latter makes possible the formation of effective and extensive hydrogen bond networks with participation of water molecules. In addition, protonated guest species and water itself can act as proton carriers, thus, boosting proton conduction (Table 2) [12][13][14].
Several 3D open-framework M(II) phosphates have been reported [14][143], which consist of [CoPO4]∞− or [Zn2(HPO4)2(H2PO4)2]2− anionic frameworks that contain organic charge-compensating ions in their internal cavities. (C2N2H10)0.5CoPO4 exhibited negligible conductivity in anhydrous conditions; however, it displayed a relatively high water-mediated proton conductivity 2.05 × 10−3 S·cm−1 at 56 °C and 98% RH. On the other hand, the solid NMe4·Zn[HPO4][H2PO4] experiences a structural transformation from monoclinic (α) to orthorhombic (β) upon heating at 149 °C. Both polymorphs contain 12-membered rings composed of tetrahedral Zn2+ ions linked to protonated phosphate groups without changing Zn-O-P connectivity (Figure 8).
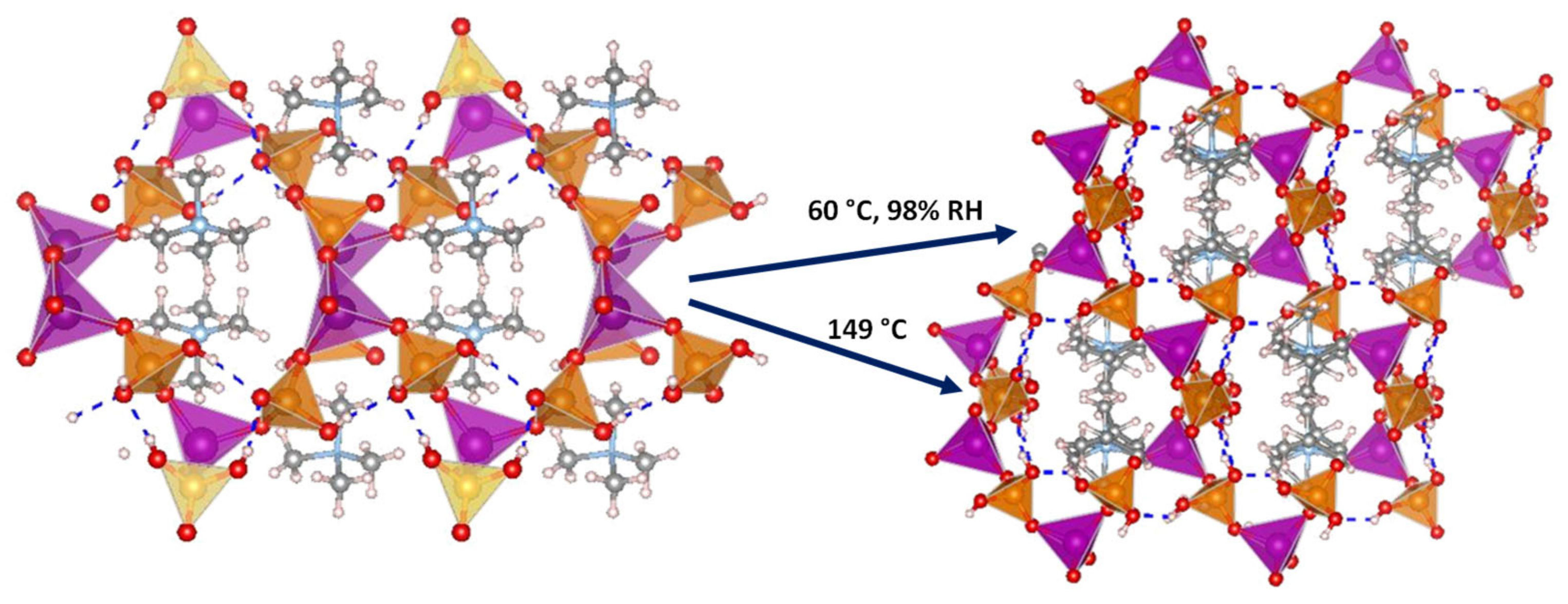
Figure 8. Irreversible structural transformation in NMe4Zn[HPO4][H2PO4]4 adapted from [143]. N (sky-blue), O (red), Zn (magenta), P (orange), C (grey) and H (pale pink) atoms.
The α phase transforms into the β phase at high humidity and temperatures above 60 °C, and then reaches a proton conductivity of 1.30 × 10−2 S·cm−1 at 98% RH, a behaviour that might be attributed to the participation of adsorbed water molecules in creating H-bonding networks with effective pathways for proton conduction. The conductivity drastically decreases at 65 °C. In anhydrous conditions, the α phase exhibited a proton conductivity of ~10−4 S·cm−1 at 160 °C, similar values were found for other reported zinc phosphates at temperatures between 130 and 190 °C [12][144][145].
Membrane-electrode assembly prepared with the pelletized solid, gave an observed open circuit voltage (OCV) of 0.92 V at 190 °C measured in a H2/air cell, suggesting that the dominant conductive species are protons, and the proton transport from anode to cathode takes place through H-bond networks in the pellet.
As an example of 2D metal phosphate water-assisted proton conductors, (C2H10N2) [Mn2(HPO4)3](H2O), displayed a proton conductivity of 1.64 × 10−3 S·cm−1 under 99% RH at 20 °C. This proton conductivity was attributed to the formation of dense H-bond networks in the lattice, composed of Mn3O13 units-containing anionic layers [146], which provide efficient proton-transfer pathways for a Grotthuss-type proton transport at high RH.
Another way of improving the proton conductivity in layered divalent metal phosphates is favouring the formation of hydrogen bond networks by ion exchange. For instance [17], partial exchange of Na+ for ethylendiammonium yielded two new crystalline phases, with composition (C2H10N2)xNa1−x[Mn2(PO4)2] (x = 0.37 y 0.54), which influenced formation of extended hydrogen bond networks and concomitant increase in proton conductivity, from 2.22 × 10−5 S·cm−1 for the as-synthesized material (in ethylendiammonium form) to 1.3 × 10−2 S·cm−1 (x = 0.37) and 2.1 × 10−2 S·cm−1 (x = 0.54) at 99% RH and 30 °C.
This strategy of enhancing proton conductivity was further extended successfully to other 2D manganese phosphates [18]. A proton conductivity value as high as 7.72 × 10−2 S·cm−1 was reached at 30 °C and 99% RH for K+-exchanged compounds, which compares well with those of MOF-based open-framework materials [147][148][149]. The 1D solid [Zn3(H2PO4)6(H2O)3](Hbim) (Hbim= benzimidazole) prepared by mechanochemical synthesis is characterized by presenting a dual-function as proton conductor.
It loses the coordinated water by heating transforming into [Zn3(HPO4)6](Hbim), which exhibits an intrinsic proton conductivity higher than the hydrated form, reaching a value of 1.3 × 10−3 S·cm−1 at 120 °C, believed to be due to a rearrangement of the conduction path and the liquid-like behaviour of benzimidazole molecules. In addition, this solid also showed porosity, thus, enabling the adsorption of gaseous methanol that further improved the proton conductivity of the anhydrous phase. This enhancement of proton conductivity in methanol-adsorbed samples was explained by the effective participation of the guest molecule in formation of extended hydrogen-bond interactions [19].
An ordered-to-disordered structural transformation and its implication in proton conduction were investigated for the 1D copper phosphate [ImH2][Cu(H2PO4)2Cl]·H2O (Im = imidazole). In this structure, the protonated imidazole (ImH2) and the water molecule are located in interspaces of the anionic chains [Cu(H2PO4)2Cl]-. Upon heating a structural transformation from an ordered crystalline state to a disordered state occurred. Highly mobile and structurally disordered H+ carriers were supposed to be responsible of the high proton conductivity 2 × 10−2 S·cm−1 at 130 °C, under anhydrous conditions [150].
A 1D zinc phosphate-based proton conductor, [Zn3(H2PO4)6(H2O)3](BTA) (BTA = 1,2,3-benzotriazole) has been reported [151] that exhibits high proton conductivity, 8 × 10−3 S·cm−1 in anhydrous glassy-state (120 °C). The glassy-state, developed via melt-quenching, was suggested to induce isotropic disordered domains that enhanced H+ dynamics and conductive interfaces. In fact, the capability of the glassy-state material as an electrolyte was found suitable for the rechargeable all-solid-state H+ battery operated in a wide range of temperatures from 25 to 110 °C.
Focus has been also put on metal phosphate-based solid solutions [152][153]. Vacancies can be generated that introduce extra protons into the structure and increase the proton conduction. This effect was investigated for the 1D rubidium and magnesium polyphosphate compound, RbMg1-xH2x(PO3)3·yH2O, for which system a maximum proton conductivity of 5.5 × 10−3 S·cm−1 was measured at 170 °C with a vehicle-type mechanism of H3O+ conduction.
The proton conductivity results from H-bond interactions between water molecules and corner-sharing PO4 chains that provide formation of sandwiched edge-sharing RbO6-MgO6 chain [152]. Another example is the solid solution with composition Co1-xZnx(H2PO4)2·2H2O (0 < x < 1.0), which showed the highest conductivity value, 2.01 × 10−2 S·cm−1 at 140 °C, for a composition Co0.5Zn0.5(H2PO4)2·2H2O [153].
4.2. Trivalent Metal Phosphates
Zeolite-like open framework metal(III) phosphates consist of metal(III) ions-phosphate species (PO43-, HPO42-, H2PO4-) linkages featuring internal cavities, where charge-compensating cations and/or neutral species are located and, thus, dense hydrogen bond networks frequently result. In addition, the robust inorganic framework endues this porous material with better thermal and chemical stability compared with porous coordination polymers/metal organic frameworks (PCPs/MOFs) [13].
Regarding to proton conductivity (Table 2), aluminium phosphate-based solids are by far the most studied compounds [154][155][156][157][158][159][160][161][162][163][164][165][166][167]. The species inside channels affect in different ways to proton conduction. Thus, while water adsorption is key to assist proton transfer in (NH4)2Al4(PO4)4(HPO4)·H2O by a hopping mechanism along H-bond chains [166], densely packed NH4+ ions show negligible contribution because of hampered migration.
By using an organic template-free synthetic methodology, a 3D open-framework aluminophosphate Na6[(AlPO4)8(OH)6]·8H2O (JU103) was prepared [158], which showed a proton conductivity of 3.59 × 10−3 S·cm−1, at 20 °C and 98% RH. It was argued that the enhanced conductivity of the as-synthesized material as compared to its NH4+- or Ag+-exchanged forms is indicative of a beneficial effect of hydrated Na+ ions in generating proton-transfer pathways. The 3D cesium silicoaluminophosphate, Cs2(Al0.875Si0.125)4(P0.875Si0.125O4)4(HPO4), belonging to the structural family of SAPOs, was shown to exhibit a remarkable proton conductivity of 1.70 × 10−4 S·cm−1 at low temperature and RH (20 °C and 30%, respectively) [168][169].
Several 2D aluminophosphates have been reported as proton conductors [154][156]. These compounds (denoted as AlPO-CJ70/2) are structurally characterized by displaying an anionic layer: [Al2P3O12]3-, formed by alternating Al3+ and phosphorus tetrahedra, which is charge-compensated by N,N-dimethylbenzylamine or α-methylbenzylamine ions, respectively. In these layered structures, extended H-bond networks are formed through interactions of the amine N atoms, H2O molecules and protruding phosphate groups of the anionic layer. Consequently, water-mediated proton conduction processes occurred upon immersion in water, with σ values around 10−3 S·cm−1, at 80 °C, and Ea values of 0.16−0.2 eV, typical of a Grotthuss-type proton-transfer mechanism.
By following a synthetic route in which methylimidazolium dihydrogenphosphate was used as a solvent, structure-directing agent, and a phosphorus source, the solid (C4H7N2)(C3H4N2)2·Al3(PO4)4·0.5H2O (SCU−2) was obtained [164]. Its layered structure, built up from corner-sharing Al3+ and PV tetrahedra, features 8-member rings where guest imidazolium ions and water molecules are hosted. At 85 °C and 98% RH, this solid showed a proton conductivity of 5.9 × 10−3 S·cm−1 and a low activation energy (0.20 eV), characteristic of a Grotthuss-type proton-transfer mechanism. An efficient pathway for the proton transfer was attributed to the hydrogen bond network established between the imidazolium ions and water molecules interacting with the host framework.
A few examples of phosphate-based proton conductors of other trivalent metals do exist. Among them, two Fe(III) phosphates, 1D (C4H12N2)1.5[Fe2(OH)(H2PO4)(HPO4)2(PO4)]·0.5H2O [13] and 3D open-framework iron(III) phosphate (NH3(CH2)3NH3)2[Fe4(OH)3(HPO4)2(PO4)3]·4H2O [170], have been reported. Both compounds contain Fe4O20 tetramers as a common structural feature. The 1D solid is composed of chains of tetramers bridged by PO43- groups and having terminal H2PO4- and HPO42- groups [171], while piperazinium cations and water molecules are disorderly situated in between chains. This arrangement gives rise to extended hydrogen bonding interactions and hence proton conducting pathways. The proton conductivity measured at 40 °C and 99% RH was 5.14 × 10−4 S·cm−1, and it was maintained upon dispersion of this solid in PVDF [13]. In the case of the 3D solid, infinite chains of interconnected tetramers are interlinked, in turn by phosphate groups that generate large tunnels (Figure 9). The diprotonated 1,3-diaminopropane and water molecules, localized inside tunnels, form an extended hydrogen bond network with the P–OH groups pointing toward cavities. These interactions favour proton hopping, the measured proton conductivity being of 8.0 × 10−4 S·cm−1, at 44 °C and 99% RH, and with an Ea of 0.32 eV [172]. Furthermore, the proton conductivity of this compound increased up to 5 × 10−2 S·cm−1 at 40 °C upon exposure to aqua-ammonia vapors from 1 M NH3·H2O solution. This result confirms this treatment as an effective way of enhancing proton conductivity, which has been elsewhere demonstrated for the case of coordination polymers [173][174]. The observed variation of Ea with the ammonia concentration also suggested that NH3, as well as H2O, molecules contribute to create proton-transfer pathways, by a Grotthuss mechanism. However, when ammonia concentrations were lower than 0.5 M, the proton conduction mechanism tended to be vehicle-type one [156].
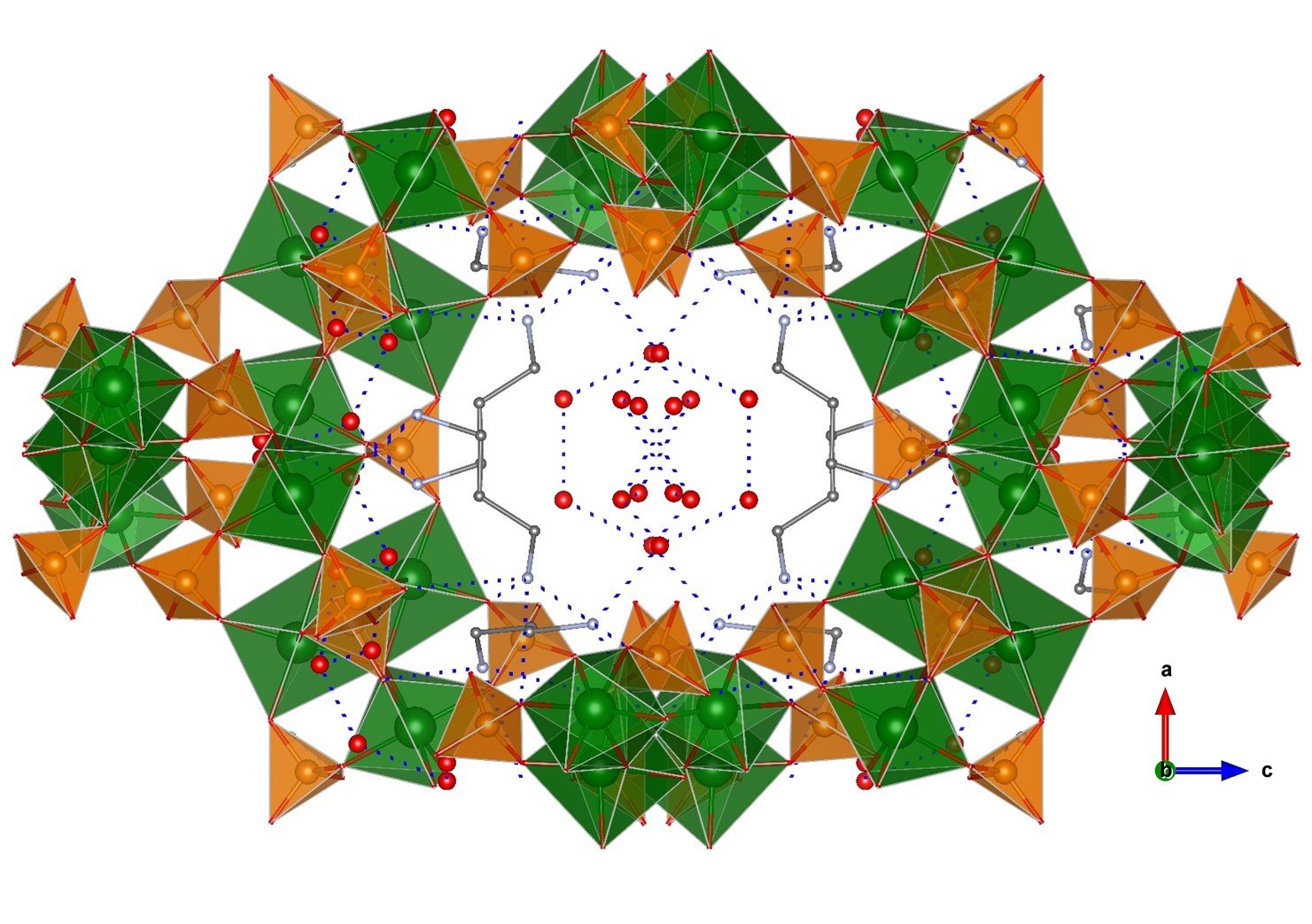
Figure 9. Open-framework structure of (NH3(CH2)3NH3)2[Fe4(OH)3(HPO4)2(PO4)3]·4H2O showing guest species inside channels and H-bond interactions. Fe (green), O (red), P (orange), and C (grey) atoms.
Table 2. Proton conductivity data for selected divalent and trivalent metal phosphates.
|
Compounds/Dimensionality |
Temperature (°C)/RH(%) |
Conductivity (S·cm-1) |
Ea (eV) |
Ref. |
|
|
Divalent Metal Phosphates |
|
|
|
|
|
|
(C2N2H10)0.5CoPO4/3D |
56/98 |
2.05 × 10-3 |
1.01 |
[143] |
|
|
NMe4·Zn[HPO4][H2PO4] (β phase)/3D |
60/98 |
1.30 × 10-2 |
0.92 |
[14] |
|
|
(C2H10N2) [Mn2(HPO4)3](H2O)/2D |
20/99 |
1.64 × 10-3 |
0.22 |
[146] |
|
|
(C2H10N2)xNa1−x[Mn2(PO4)2]/2D |
30/99 |
2.1 × 10-2 |
0.14 |
[17] |
|
|
(C2H10N2)1-xKx[Mn2(PO4)2]·2H2O/2D |
30/99 |
7.72 × 10-2 |
0.18 |
[18] |
|
|
(C2H10N2)1-xKx[Mn2(HPO4)3] (H2O)/2D |
30/99 |
0.85 × 10-2 |
0.081 |
[18] |
|
|
[Zn3(H2PO4)6(H2O)3](Hbim)/1D |
120 |
|
1.3 × 10−3 |
0.50 |
[19] |
|
[ImH2][Cu(H2PO4)2Cl]·H2O/1D |
130 |
|
2.0 × 10−2 |
0.1 |
[150] |
|
[Zn3(H2PO4)6(H2O)3](BTA)/1D |
120 |
|
8.0 × 10−3 |
0.39 |
[151] |
|
RbMg0.9H0.2(PO3)3·yH2O/1D |
170 |
|
5.5 × 10−3 |
--- |
[152] |
|
Co0.5Zn0.5(H2PO4)2·2H2O/1D |
140 |
|
2.01×10−2 |
--- |
[153] |
|
Trivalent Metal Phosphates |
|
|
|
|
|
|
Na6[(AlPO4)8(OH)6]·8H2O/3D |
20/98 |
|
3.59×10-3 |
0.21 |
[158] |
|
[C9H14N]8[H2O]4·[Al8P12O48H4]/2D |
80, in water |
|
9.25×10-4 |
0.16 |
[154] |
|
[R-,S-C8H12N]8[H2O]2·[Al8P12O48H4]/2D |
90/98 |
|
3.01×10-3 |
0.20 |
[156] |
|
(C4H7N2)(C3H4N2)2·Al3(PO4)4·0.5H2O/2D |
85/98 |
|
5.94×10-3 |
0.20 |
[164] |
|
In(HPO4)(H2PO4)(D,L-C3H7NO2)/3D |
85/98 |
|
2.9 × 10-3 |
0.19 |
[16] |
|
(NH3(CH2)3NH3)2[Fe4(OH)3(HPO4)2(PO4)3]· 4H2O/1D |
40/99 |
|
5.0 × 10-2 |
--- |
[170] |
Hbim = benzimidazole; Im =imidazole; BTA = 1,2,3-benzotriazole.
For the series of isostructural imidazole cation (ImH2)-templated layered metal phosphates, [ImH2][X-(HPO4)2(H2O)2] (FJU−25-X, X = Al, Ga, and Fe), it was found that the proton conductivity was dependent on mobility of imidazole guests, FJU−25-Fe exhibiting the highest proton conductivity (5.21 × 10−4 S·cm−1 at 90 °C). The determined activation energies (~0.20 eV) were indicative of a Grotthuss-type mechanism of proton conduction.
The amino acid-template indium phosphate, In(HPO4)(H2PO4)(D,L-C3H7NO2) (SCU−12), represents a singular example of 3D metal phosphate-based proton conductors [16].
Its crystal structure is formed by edge-sharing four-ring ladders, with the amino acid molecules attached to the ladders through In–O bonds. Further bridging the indium phosphate ladders by the H2PO4- groups gives rise to a three-dimensional structure. The presence of two kinds of proton carriers, H2PO4- ions and zwitterionic alanine molecules, favours the development of high proton conductivities (2.9 × 10−3 S·cm−1 at 85 °C and 98% RH) through a Grotthuss-type proton-transfer mechanism (Ea = 0.19 eV).
Other trivalent metal phosphates have been reported, e.g., BPOx [175] and CePO4 [176]. The former exhibited a proton conductivity of 7.9 × 10−2 S·cm−1 as self-supported electrolyte and 4.5 × 10−2 S·cm−1 as (PBI)−4BPOx composite membrane, measured at 150 °C and 5% RH, but structure/conductivity correlations were not established because of its amorphous nature. The latter showed a low-temperature (RT) proton conduction < 10−5 S·cm−1 at 100% RH through a structure-independent proton-transport mechanism [176].
There are only a few examples of metal(III) pyrophosphates displaying proton conductivity. Among them is the open framework magnesium aluminophosphate MgAlP2O7(OH)(H2O)2 (JU102) [167]. Its structure is composed of tetrahedral Al3+ and octahedral Mg2+ ions coordinated by pyrophosphate ions. This connectivity results in an open framework with unidirectional 8-ring channels. The proton conduction properties originate from the existence of an H-bond network in which coordinated water molecules participate.
Thus, the proton conductivity measured at 55 °C on water-immersed samples was 3.86 × 10−4 S·cm−1 [167], which raised to 1.19 × 10−3 S·cm−1 when calcined at 250 °C and measured at the same conditions, while the Ea value hardly changed from 0.16 to 0.2 eV. This behaviour was explained as being due to a dehydration–rehydration process that enhances proton conductivity by altering the H-bonding network and the pathway of proton transfer.
Another example of 3D open framework metal(III) pyrophosphate is the compound NH4TiP2O7 [177]. The structure of this solid is composed of negatively charged [TiP4O12]− layers, forming one-dimensional six-membered ring channels, where the NH4+ ions are located. Its proton conductivity increased from 10−6 S·cm−1 under anhydrous conditions to 10−3 S·cm−1 at full-hydration conditions and 84 °C. The low Ea value, 0.17 eV, characteristic of a Grotthuss-type proton-transfer mechanism, was associated with the role played by the NH4+ ions in the channels as proton donors and promoters of proton migration. A drop in proton conductivity was observed when the triclinic TiP2O7 phase formed by thermal decomposition of NH4TiP2O7 [177].
5. Outlook
Great efforts have been devoted to the synthesis and characterization of metal phosphate-based proton conductors over more than three decades. Among them, zirconium phosphates are prominent not only because of the feasibility of Zr(IV) and phosphate ions to form a rich variety of crystallographic architectures (from 1D to 3D open frameworks) but also due to their outstanding properties and workability while being environmentally benign and low cost materials.
Although the prototype layered α-zirconium phosphate has been commonly proven as a filler for PEMFCs devices, new synthetic designs of M(IV) phosphates, including pyrophosphate compounds, are promising candidates to broaden their applicability in different electrochemical devices. Other M(IV) phosphates and pyrophosphates (M = Ti, Sn) are less known due, in part, to their amorphous nature or strong tendency to amorphise at working temperatures, though, in some cases, these compounds presented remarkable proton conductivity properties.
CsH2PO4, a super protonic material, was proven as a suitable electrolyte for both H2/O2 and direct methanol fuel cells operated at ~240 °C, and provides excellent performance when controlling its thermal stability. In addition, combinations with other materials are possible to adjust the specific characteristics of the composite CsH2PO4 electrolyte, thus, offering a wide range of compositions with tuning properties.
Recently, research and developments in metal phosphate proton conductors have been addressed to divalent or trivalent metal phosphates, which present a remarkable structural versatility and tunable conductivities; however, more in-depth studies are required to assess their potential use and applicability for low and intermediate temperature fuel cells.
Applications of metal phosphates as electrolytes or as electrolyte components are in continuous progress, although their use for energy storage and conversion remains a challenge. For practical applications in fuel cells at low/intermediate temperatures, phosphate-based proton conducting electrolytes have demonstrated acceptable proton conductivity values; however, other features, such as their mechanical strength, chemical/thermal stabilities, film-forming ability (in the case of composite membranes), durability, and fuel cross-over, are key factors to be improved.
Funding: This research was funded by PID2019−110249RB-I00 (MICIU/AEI, Spain) and PY20−00416 (Junta de Andalucia, Spain/FEDER) research projects.
Acknowledgments: M.B.G. thanks PAIDI2020 research grant (DOC_00272 Junta de Andalucia, Spain) and R.M.P.C. thanks University of Malaga under Plan Propio de Investigación for financial support.
This entry is adapted from the peer-reviewed paper 10.3390/ma15041292
References
- Shivhare, A.; Kumara, A.; Srivastava, R. Metal phosphate catalysts to upgrade ligno-cellulose biomass into value-added chemicals and biofuels. Green Chem. 2021, 23, 3818–3841.
- Zhao, H.; Yuan, Z.-Y. Insights into Transition Metal Phosphate Materials for Efficient Electrocatalysis. ChemCatChem 2020, 12, 3797–3810.
- Chen, L.; Zhao, Y.; Yang, J.; Liu, D.; Wei, X.; Wang, X.; Zheng, Y. New Versatile Synthetic Route for the Preparation of Metal Phosphate Decorated Hydrogen Evolution Photocatalysts. Inorg. Chem. 2020, 59, 1566–1575.
- Goñi-Urtiaga, A.; Presvytes, D.; Scott, K. Solid acids as electrolyte materials for proton exchange membrane (PEM) electrol-ysis: Review. Int. J. Hydrog. Energy 2012, 37, 3358–3372.
- Paschos, O.; Kunze, J.; Stimming, U.; Maglia, F. A review on phosphate based, solid state, protonic conductors for interme-diate temperature fuel cells. J. Phys. Condens. Matter 2011, 23, 234110.
- Haile, S.M.; Chisholm, C.R.I.; Sasaki, K.; Boysen, D.A.; Uda, T. Solid acid proton conductors: From laboratory curiosities to fuel cell electrolytes. Faraday Discuss. 2007, 134, 17–39.
- Cheng, Q.; Zhao, X.; Yang, G.; Mao, L.; Liao, F.; Chen, F.; He, P.; Pan, D.; Chen, S. Recent advances of metal phosphates-based electrodes for high-performance metal ion batteries. Energy Storage Mater. 2021, 41, 842–882.
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the fu-ture? Mater. Today 2015, 19, 69–87.
- Mohammad, N.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S. A review on synthesis and characterization of solid acid mate-rials for fuel cell applications. J. Power Sources 2016, 322, 77–92.
- Pica, M.; Donnadio, A.; Casciola, M. From microcrystalline to nanosized α-zirconium phosphate: Synthetic approaches and applications of an old material with a bright future. Coord. Chem. Rev. 2018, 374, 218–235.
- Wong, N.E.; Ramaswamy, P.; Lee, A.S.; Gelfand, B.S.; Bladek, K.J.; Taylor, J.M.; Spasyuk, D.M.; Shimizu, G.K.H. Tuning In-trinsic and Extrinsic Proton Conduction in Metal−Organic Frameworks by the Lanthanide Contraction. J. Am. Chem. Soc. 2017, 139, 14676–14683.
- Horike, S.; Umeyama, D.; Inukai, M.; Itakura, T.; Kitagawa, S. Coordination-network-based ionic plastic crystal for anhy-drous proton conductivity. J. Am. Chem. Soc. 2012, 134, 7612–7615.
- Zhang, K.-M.; Lou, Y.-L.; He, F.-Y.; Duan, H.-B.; Huang, X.-Q.; Fan, Y.; Zhao, H.-R. The water-mediated proton conductivity of a 1D open framework inorganic-organic hybrid iron phosphate and its composite membranes. Inorg. Chem. Commun. 2021, 134, 109032.
- Yu, J.-W.; Yu, H.-J.; Ren, Q.; Zhang, J.; Zou, Y., Luo, H.-B.; Wang, L.; Ren, X.-M. Humidity-sensitive irreversible phase trans-formation of open-framework zinc phosphate and its water-assisted high proton conduction properties. Dalton Trans. 2021, 50, 8070–8075.
- Su, X.; Yao, Z.; Ye, Y.; Zeng, H.; Xu, G.; Wu, L.; Ma, X.; Chen, Q.-H.; Wang, L.; Zhang, Z.; et al. 40-Fold Enhanced Intrinsic Proton Conductivity in Coordination Polymers with the Same Proton-Conducting Pathway by Tuning Metal Cation Nodes. Inorg. Chem. 2016, 55, 983–986.
- Shi, J.; Wang, K.; Li, J.; Zeng, H.; Zhang, Q.; Lin, Z. Exploration of new water stable proton-conducting materials in an ami-no acid-templated metal phosphate system. Dalton Trans. 2018, 47, 654–658.
- Zhang, K.-M.; He, F.-Y.; Duan, H.-B.; Zhao, H.-R. An alkali metal ion-exchanged metal-phosphate (C2H10N2)xNa1−x[Mn2(PO4)2] with high proton conductivity of 10−2 S·cm−1. Inorg. Chem. 2019, 58, 6639–6646.
- Zhang, K.-M.; Jia, Y.; Gu, Y., He, F.-Y.; Zhao, H.-R. A facile and efficient method to improve the proton conductivity of open framework metal phosphates under aqueous condition. Inorg. Chem. Commun. 2020, 120, 108128.
- Umeyama, D.; Horike, S.; Inukai, M.; Kitagawa, S. Integration of Intrinsic Proton Conduction and Guest-Accessible Nano-space into a Coordination Polymer. J. Am. Chem. Soc. 2013, 135, 11345–11350.
- Baranov, A.I.; Khiznichenko, V.P.; Sandler, V.A.; Shuvalov, L.A. Frequency dielectric dispersion in the ferroelectric and superionic phases of CsH2PO4. Ferroelectrics 1988, 81, 183–186.
- Colomban, P. Chemistry of Solid-State Materials. In Proton Conductors: Solid, Membranes and Gels-Materials and Devices; Cam-bridge University Press: Cambridge, UK, 1992.
- Fragua, D.M.; Castillo, J.; Castillo, R.; Vargas, R.A. New amorphous phase KnH2PnO3n+1(n>>1) in KH2PO4. Rev. Latin Am. Metal. Mat. 2009, 2, 491–497.
- Skou, E.; Andersen, I.G.K.; Simonsen, K.E.; Andersen, E.K. Is UO2HPO4·4H2O a proton conductor? Solid State Ion. 1983, 9, 1041–1047.
- Cabeza, A.; Martinez, M.; Benavente, J.; Bruque, S. Current rectification by H3OUO2PO4 3H2O (HUP) thin films in electrolyte media. Solid State Ion. 1992, 51, 127–131.
- Barboux, P.; Morineau, R.; Livage, J. Protonic conductivity in hydrates. Solid State Ion. 1988, 27, 221–225.
- Barboux, P.; Livage, J. Ionic conductivity in fibrous Ce(HPO4)2·(3+x)H2O. Solid State Ion. 1989, 34, 47–52.
- Li, J.; Yi, M.; Zhang, L.; You, Z.; Liu, X.; Li, B. Energy related ion transports in coordination polymers. Nano Select. 2021, 1–19.
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352.
- Wei, J. Proton-Conducting Materials Used as Polymer Electrolyte Membranes in Fuel Cells (ch 9). In Polymer-Based Multi-functional Nanocomposites and Their Applications; Song, K., Liu, C., Guo, J.Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 9, pp. 245–260.
- Sazali, N.; Salleh, W.N.W.; Jamaludin, A.S.; Razali, M.N.M. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99.
- Dupuis, A.-C. Proton exchange membranes for fuel cells operated at medium temperatures: Materials and experimental techniques. Prog. Mater. Sci. 2011, 56, 289–327.
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384.
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585.
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange mem-branes (PEMs). Chem. Rev. 2004, 104, 4587–4612.
- Han, X.; Xie, Y.; Liu, D.; Chen, Z.; Zhang, H.; Pang, J.; Jiang, Z. Synthesis and properties of novel poly(arylene ether)s with densely sulfonated units based on carbazole derivative. J. Membr. Sci. 2019, 589, 117230.
- Kuzmenko, M.; Poryadchenko, N. Perspective materials for application in fuel-cell technologies. In Fuel Cell Technologies: State and Perspectives; Sammes. N., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 253–258.
- Lee, K.-S.; Maurya, S.; Kim, Y.S.; Kreller, C.R.; Wilson, M.S.; Larsen, D.; Elangovan, S.E.; Mukundan, R. Intermediate temper-ature fuel cells via an ion-pair coordinated polymer electrolyte. Energy Env. Sci. 2018, 11, 979–987.
- Norby, T. Solid-state protonic conductors: Principles, properties, progress and prospects. Solid State Ion. 1999, 125, 1–11.
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. Approaches and Recent Development of Polymer Electrolyte Membranes for Fuel Cells Operating above 100 °C. Chem. Mater. 2003, 15, 4896–4915.
- Loreti, G.; Facci, A.L.; Ubertini, S. High-Efficiency Combined Heat and Power through a High-Temperature Polymer Elec-trolyte Membrane Fuel Cell and Gas Turbine Hybrid System. Sustainability 2021, 13, 12515.
- Boysen, D.A.; Uda, T.; Chisholm, C.R.I.; Haile, S.M. High-Performance Solid Acid Fuel Cells Through Humidity Stabiliza-tion. Science 2004, 303, 68–70.
- Zhang, J.; Aili, D.; Lu, S.; Li, Q.; Jiang, S.P. Advancement toward Polymer Electrolyte Membrane Fuel Cells at Elevated Temperatures. AAAS Res. 2020, 2020, 9089405.
- Clearfield, A.; Smith, S.D. The crystal structure of zirconium phosphate and the mechanism of its ion exchange behavior. J. Colloid Interface Sci. 1968, 28, 325–330.
- Clearfield, A.; Smith, G.D. Crystallography and structure of alpha-zirconium bis(monohydrogen orthophosphate) mono-hydrate. Inorg. Chem. 1969, 8, 431–436.
- Troup, J.M.; Clearfield, A. Mechanism of ion exchange in zirconium phosphates. 20. Refinement of the crystal structure of .alpha.-zirconium phosphate. Inorg. Chem. 1977, 16, 3311–3314.
- Casciola, M. From layered zirconium phosphates and phosphonates to nanofillers for ionomeric membranes. Solid State Ion. 2019, 336, 1–10.
- Ogawa, T.; Ushiyam, H.; Lee, J.-M.; Yamaguchi, T.; Yamashita, K. Theoretical Studies on Proton Transfer among a High Density of Acid Groups: Surface of Zirconium Phosphate with Adsorbed Water Molecules. J. Phys. Chem. C 2011, 115, 5599–5606.
- Alberti, G.; Casciola, M.; Costantino, U. Inorganic ion-exchange pellicles obtained by delamination of α-zirconium phos-phate crystals. J. Colloid Interface Sci. 1985, 107, 256–263.
- Alberti, G.; Casciola, M.; Costantino, U.; Leonardi, M. AC conductivity of anhydrous pellicular zirconium phosphate in hydrogen form. Solid State Ion. 1984, 14, 289–295.
- Casciola, M.; Costantino, U.; D’Amico, S. Protonic conduction of intercolation compounds of α-zirconium phosphate with propylamine. Solid State Ion. 1986, 22, 127–133.
- Alberti, G.; Casciola, M.; Cavalaglio, S.; Vivani, R. Proton conductivity of mesoporous zirconium phosphate pyrophos-phate. Solid State Ion. 1999, 125, 91–97.
- Alberti, G.; Casciola, M.; Donnadio, A.; Piaggio, P.; Pica, M.; Sisani, M. Preparation and characterisation of α-layered zirco-nium phosphate sulfophenylenphosphonates with variable concentration of sulfonic groups. Solid State Ion. 2005, 176, 2893–2898.
- Gui, D.; Zheng, T.; Xie, J.; Cai, Y.; Wang, Y.; Chen, L.; Diwu, J.; Chai, Z.; Wang, S. Significantly dense two-dimensional hy-drogen-bond network in a layered zirconium phosphate leading to high proton conductivities in both water-assisted low-temperature and anhydrous intermediate-temperature regions. Inorg. Chem. 2016, 55, 12508–12511.
- Yu, J.-W.; Yu, H.-J.; Yao, Z.-Y.; Li, Z.-H.; Ren, Q.; Luo, H.-B.; Zou, Y.; Wang, L.; Ren, X.-M. A water-stable open-framework zirconium(IV) phosphate and its water-assisted high proton conductivity. CrystEngComm 2021, 23, 6093–6097.
- Gui, D.; Dai, X.; Tao, Z.; Zheng, T.; Wang, X.; Silver, M.A.; Shu, J.; Chen, L.; Wang, Y.; Zhang, T.; et al. Unique proton trans-portation pathway in a robust inorganic coordination polymer leading to intrinsically high and sustainable anhydrous proton conductivity. J. Am. Chem. Soc. 2018, 140, 6146–6155.
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104.
- Alberti, G.; Casciola, M.; Capitani, D.; Donnadio, A.; Narducci, R.; Pica, M.; Sganappa, M. Novel Nafion–zirconium phos-phate nanocomposite membranes with enhanced stability of proton conductivity at medium temperature and high relative humidity. Electrochim. Acta 2007, 52, 8125–8132.
- Grot, W.G.; Rajendran, G.; Hendrickson, J.S. International Patent Application No. PCT/US96/03804, International Publication No. WO 96/2975, 26, September, 1996.
- Alberti, G.; Casciola, M.; Pica, M.; Tarpanelli, T.; Sganappa, M. New Preparation Methods for Composite Membranes for Medium Temperature Fuel Cells Based on Precursor Solutions of Insoluble Inorganic Compounds. Fuel Cells 2005, 5, 366–374.
- Casciola, M.; Bagnasco, G.; Donnadio, A.; Micoli, L.; Pica, M.; Sganappa, M.; Turco, M. Conductivity and Methanol Permea-bility of Nafion–Zirconium Phosphate Composite Membranes Containing High Aspect Ratio Filler Particles. Fuel Cells 2009, 9, 394–400.
- Arbizzani, C.; Donnadio, A.; Pica, M.; Sganappa, M.; Varzi, A.; Casciola, M.; Mastragostino, M. Methanol permeability and performance of Nafion–zirconium phosphate composite membranes in active and passive direct methanol fuel cells. J. Power Sources 2010, 195, 7751–7756.
- Pica, M.; Donnadio, A.; Casciola, M.; Cojocaru, P.; Merlo, L. Short side chain perfluorosulfonic acid membranes and their composites with nanosized zirconium phosphate: Hydration, mechanical properties and proton conductivity. J. Mater. Chem. 2012, 22, 24902–24908.
- Pica, M.; Donnadio, A.; Capitani, D.; Vivani, R.; Troni, E.; Casciola, M. Advances in the Chemistry of Nanosized Zirconium Phosphates: A New Mild and Quick Route to the Synthesis of Nanocrystals. Inorg. Chem. 2011, 50, 11623–11630.
- Bauer, F.; Willert-Porada, M. Comparison between Nafion and a Nafion zirconium phosphate nanocomposite in fuel cell applications. Fuel Cells 2006, 6, 261–269.
- Casciola, M.; Cojocaru, P.; Donnadio, A., Giancola, S.; Merlo, L.; Nedellec, Y.; Pica, M.; Subianto, S. Zirconium phosphate reinforced short side chain perflurosulfonic acid membranes for medium temperature proton exchange membrane fuel cell application. J. Power Sources 2014, 262, 407–413.
- Yang, C.; Srinivasan, S.; Aricò, A.S.; Creti, P.; Baglio, V.; Antonucci, V. Composition Nafion/zirconium phosphate mem-branes for direct methanol fuel cell operation at high temperature. Electrochem. Solid-State Lett. 2001, 4, A31–A34.
- Yang, C.; Costamagna, P.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Approaches and technical challenges to high tempera-ture operation of proton exchange membrane fuel cells. J. Power Sources 2001, 103, 1–9.
- Costamagna, P.; Yang, C.; Bocarsly, A.B.; Srinivasan, S. Nafion (R) 115/zirconium phosphate composite membranes for op-eration of PEMFCs above 100 °C. Electrochim. Acta 2002, 47, 1023–1033.
- Yang, C.; Srinivasan, S.; Bocarsly, A.B.; Tulyani, S.; Benziger, J.B. A comparison of physical properties and fuel cell perfor-mance of Nafion and zirconium phosphate/Nafion composite membranes. J. Membr. Sci. 2004, 237, 145–161.
- Lee, H.-K.; Kim, J.-I.; Park, J.-H.; Lee, T.-H. A study on self-humidifying PEMFC using Pt-ZrP-Nafion composite membrane. Electrochim. Acta 2004, 50, 761–768.
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton Conductivity of Composite Polyelectrolyte Membranes with Metal-Organic Frameworks for Fuel Cell Applications. Adv. Mater. Interfaces 2019, 6, 1801146.
- Liu, K.L.; Lee, H.C.; Wang, B.Y.; Lue, S.J.; Lu, C.Y.; Tsai, L.D.; Fang, J.; Chao, C.Y. Sulfonated poly(styrene-block-(ethylene-ran-butylene)-block-styrene (SSEBS)-zirconium phosphate) (ZrP) composite membranes for direct methanol fuel cells. J. Membr. Sci. 2015, 495, 110–120.
- Hu, H.; Ding, F.; Ding, H.; Liu, J.; Xiao, M.; Meng, Y.; Sun, L. Sulfonated poly(fluorenyl ether ketone)/Sulfonated α-zirconium phosphate Nanocomposite membranes for proton exchange membrane fuel cells. Adv. Compos. Hybrid Mater. 2020, 3, 498–507.
- Pandey, J.; Seepana, M.M.; Shukla, A. Zirconium phosphate based proton conducting membrane for DMFC application. Int. J. Hydrog. 2015, 40, 9410–9421.
- He, R.; Li, Q.; Xiao, G.; Bjerrum, N.J. Proton conductivity of phosphoric acid doped polybenzimidazole and its composites with inorganic proton conductors. J. Membr. Sci. 2003, 226, 169–184.
- Gouda, M.H.; Tamer, T.M.; Konsowa, A.H.; Farag, H.A.; Mohy Eldin, M.S. Organic-Inorganic Novel Green Cation Exchange Membranes for Direct Methanol Fuel Cells. Energies 2021, 14, 4686.
- Alberti, G.; Cardini-Galli, P.; Costantino, U.; Torracca, E. Crystalline insoluble salts of polybasic metals—I Ion-exchange properties of crystalline titanium phosphate. J. Inorg. Nucl. Chem. 1967, 29, 571–578.
- Christensen, A.N.; Anderson, E.K.; Andersen, I.G.; Alberti, G.; Nielsen, M.; Lehmann, E.K. X-Ray Powder diffraction study of layer compounds. The crystal structure of α-Ti (HPO4)2·H2O and a proposed structure for γ-Ti (H2PO4)(PO4)·2H2O. Acta Chem. Scand. 1990, 44, 865–872.
- Li, Y.J.; Whittingham, M.S. Hydrothermal synthesis of new metastable phases: Preparation and intercalation of a new lay-ered titanium phosphate. Solid State Ion. 1993, 63, 391–395.
- Kőrösi, L.; Papp, S.; Dékány, I. A layered titanium phosphate Ti2O3(H2PO4)2·2H2O with rectangular morphology: Synthesis, structure, and cysteamine intercalation. Chem. Mater. 2010, 22, 4356–4363.
- Ekambaram, S.; Serre, C.; Férey, G.; Sevov, S.C. Hydrothermal synthesis and characterization of an ethylenedia-mine-templatedmixed-valence titanium phosphate. Chem. Mater. 2000, 12, 444–449.
- Krogh Andersen, A.M.; Norby, P.; Hanson, J.C.; Vogt, T. Preparation and characterization of a new 3-dimensional zirconi-um hydrogen phosphate, τ-Zr(HPO4)2. Determination of the complete crystal structure combining synchrotron X-ray sin-gle-crystal diffraction and neutron powder diffraction. Inorg. Chem. 1998, 37, 876–881.
- Mileo, P.G.M.; Kundu, T.; Semino, R.; Benoit, V.; Steunou, N.; Llewellyn, P.L.; Serre, C.; Maurin, G.; Devautour-Vinot, S. Highly Efficient Proton Conduction in a Three-Dimensional Titanium Hydrogen Phosphate. Chem. Mater. 2017, 29, 7263–7271.
- Bortun, A.I.; Khainakov, S.A.; Bortun, L.N.; Poojary, D.M.; Rodriguez, J.; Jose, R. Garcia, J.R.; Clearfield, A. Synthesis and characterization of two novel fibrous titanium phosphates Ti2O(PO4)2·2H2O. Chem. Mater. 1997, 9, 1805–1811.
- Salvadó, M.A.; Pertierra, P.; García-Granda, S.; García, J.R.; Fernández-Diaz, M.T.; Dooryhee, E. Crystal structure, including H-atom positions, of Ti2O(PO4)2·(H2O)2 determined from synchrotron X-ray and neutron powder data. Eur. J. Solid State In-org. Chem. 1997, 34, 1237–1247.
- Babaryk, A.A.; Adawy, A.; García, I. Trobajo, C.; Amghouz, Z.; Colodrero, R.M.P. Cabeza, A.; Olivera-Pastor, P.; Ba-zaga-García, M.; dos Santos-Gómez, L. Structural and proton conductivity studies of fibrous π-Ti2O(PO4)2·2H2O: Application in chitosan-based composite membranes. Dalton Trans. 2021, 50, 7667–7677.
- Bruque, S.; Aranda, M.A.G.; R. Losilla, E.; Olivera-Pastor, P.; Maireles-Torres, P. Synthesis optimization and crystal struc-tures of layered metal(IV) hydrogen phosphates, .alpha.-M(HPO4)2.cntdot.H2O (M = Ti, Sn, Pb). Inorg. Chem. 1995, 34, 893–899.
- Huang, W.; Komarneni, S.; Noh,Y.D.; Ma, J.; Chen, K.; Xue, D.; Xuea, X.; Jiang, B. Novel inorganic tin phosphate gel: Multi-functional material. Chem. Commun. 2018, 54, 2682–2685.
- Ansari, Y.; Telpriore, G.; Tucker, C.; Angell, A. A novel, easily synthesized, anhydrous derivative of phosphoric acid for use in electrolyte with phosphoric acid-based fuel cells. J. Power Sources 2013, 237, 47–51.
- Moshareva, M.A.; Novikova, S.A.; Yaroslavtsev, A.B. Synthesis and ionic conductivity of (NH4)1–x Hx Hf2(PO4)3 (x = 0–1) NASICON-type materials. Inorg. Mater. 2016, 52, 1283–1290.
- Clearfield, A. Structural concepts in inorganic proton conductors. Solid State Ion. 1991, 46, 34–43.
- Clearfield, A.; Roberts, B.D.; Subramanian, M.A. Preparation of (NH4)Zr2(PO4)3 and HZr2(PO4)3. Mater. Res. Bull. 1984, 19, 219–226.
- Komorowski, P.G.; Agryropoulos, S.A.A.; Canaday, J.D.; Kuriakose, A.K.; Wheat, T.A.; Ahmad, A.; Gulens, J. The study of hydronium NASICON conductivity with deuterium. Solid State Ion. 1992, 50, 253–258.
- Stenina, I.A.; Kislitsyn, M.N.; Ghuravlev, N.A.; Yaroslavtsev, A.B. Phase transitions and ionic mobility in hydrogen zirco-nium phosphates with the NASICON structure, H1 ± xZr2–xMx(PO4)3⋅H2O, M = Nb, Y. Mater. Res. Bull. 2008, 43, 377–383.
- Stenina, A.; Pinus, I.Y.; Rebrov, A.I.; Yaroslavtsev, A.B. Lithium and hydrogen ions transport in materials with NASICON structure. Solid State Ion. 2004, 175, 445–449.
- Stenina, I.A.; Zhizhin, M.G.; Lazoryak, B.I.; Yaroslavtsev, A.B. Phase transitions, structure and ion conductivity of zirconium hydrogen phosphates, H1 ± xZr2–xMx(PO4)3 ⋅ H2O (M = Nb, Y). Mater. Res. Bull. 2009, 44, 1608–1612.
- Mieritz, D.; Davidowski, S.K.; Seo, D. Accessing alkali-free NASICON-type compounds through mixed oxoanion sol–gel chemistry: Hydrogen titanium phosphate sulfate, H1−xTi2(PO4)3−x(SO4)x (x=0.5–1). J. Solid State Chem. 2016, 242, 116–125.
- Sato, Y.; Shen, Y.; Nishida, M.; Kanematsu, W.; Hibino, T. Proton Conduction in Non-Doped and Acceptor-Doped Metal Pyrophosphate (MP2O7) Composite Ceramics at Intermediate Temperatures. J. Mater. Chem. 2012, 22, 3973–3981.
- Singh, B.; Kima, J.-H.; Park, J.-Y.; Song, S.-J. Dense composite electrolytes of Gd3+-doped cerium phosphates for low-temperature proton-conducting ceramic-electrolyte fuel cells. Ceram. Inter. 2015, 41, 4814–4821.
- Nagao, M.; Kamiya, T.; Heo, P.; Tomita, A.; Hibino, T.; Sano, M. Proton conduction in In3+-doped SnP2O7 at intermediate temperatures. J. Electrochem. Soc. 2006, 153, 1604–1609.
- Nagao, M.; Takeuchi, A.; Heo, P.; Hibino, T.; Sano, M.; Tomita, A. A proton-conducting In3 + -doped SnP2O7 electrolyte for intermediate-temperature fuel cells. Electrochem. Solid-State Lett. 2006, 9, A105.
- Jin, Y.; Fujiwara, K.; Hibino, T. High temperature, low humidity proton exchange membrane based on an inorganic–organic hybrid structure. Electrochem. Solid-State Lett. 2010, 13, B8.
- Jin, Y.; Shen, Y.; Hibino, T. Proton conduction in metal pyrophosphates (MP2O7) at intermediate temperatures. J. Mater. Chem. 2010, 20, 6214–6217.
- Chen, X.; Wang, C.; Payzant, E.; Xia, C.; Chu, D. An oxide ion and proton co-ion conducting Sn0.9In0.1P2O7 electrolyte for intermediate-temperature fuel cells. J. Electrochem. Soc. 2008, 155, B1264.
- Kreller, C.R.; Pham, H.H.; Wilson, M.S.; Mukundan, R.; Henson, N.; Sykora, M.; Hartl, M.; Daemen, L.; Garzon, F.H. Intra-granular phase proton conduction in crystalline Sn1−xInxP2O7 (x = 0 and 0.1). J. Phys. Chem. C 2017, 121, 23896–23905.
- Kreller, C.R.; Wilson, M.S.; Mukundan, R.; Brosha, E.L.; Garzon, F.H. Stability and conductivity of In3+-doped SnP2O7 with varying phosphorous to metal ratios. ECS Electrochem. Lett. 2013, 2, F61−F63.
- Foran, G.Y.; Goward, G.R. Site-Specific Proton Dynamics in Indium-Doped Tin Pyrophosphate. J. Phys. Chem. C 2020, 124, 28407–28416.
- Scott, K.; Xu, C.; Wu, X. Intermediate temperature proton-conducting membrane electrolytes for fuel cells. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 24–41.
- Anfimova, T.; Lie-Andersena, T.; Pristed Jensen, E.; C. Brorson Prag, C.; Nielsen, U.G.; Sørensen, D.R.; Skou, E.M.; Christen-sen, E.; Bjerrum, N.J.;.Li, Q. The effect of preparation method on the proton conductivity of indium doped tin pyrophos-phates. Solid State Ion. 2015, 278, 209–216.
- Li, W.; Bose, A.B.; Rusakova, I.A. An approach for restoring the proton conductivity of sintered tin pyrophosphate mem-branes for intermediate temperature fuel cells. J. Power Sources 2016, 307, 146–151.
- Ramaiyan, K.P.; Herrera, S.; Workman, M.J.; Semelsberger, T.A.; Atanasov, V.; Kerres, J.; Sandip Maurya, S.; Kim, Y.S.; Krel-ler, C.R.; Mukundan, R. Role of phosphate source in improving the proton conductivity of tin pyrophosphate and its com-posite electrolytes. J. Mater. Chem. A 2020, 8, 16345–16354.
- Wu, X.; Mamlouk, M.; Scott, K. A PBI-Sb0.2Sn0.8P2O7-H3PO4 Composite Membrane. Fuel Cells 2011, 11, 620–625.
- Jin, Y.C.; Nishida, M.; Kanematsu, W.; Hibino, T. An H3PO4-doped polybenzimidaz-ole/Sn0.95Al0.05P2O7 composite mem-brane for high-temperature proton exchange mem-brane fuel cells. J. Power Sources 2011, 196, 6042–6047.
- Huang, M.; Huang, X.; Deng, Y.; Fei, M.; Xu, C.; Cheng, J. Graphite oxide-incorporated SnP2O7 solid composite electrolyte for high-temperature proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2017, 42, 1113–1119.
- Le, M.-V.; Tsai, D.-S.; Yang, C.-Y.; Chung, W.-H.; Lee, H.-Y. Proton conductors of cerium pyrophosphate for intermediate temperature fuel cell. Electrochim. Acta 2011, 56, 6654–6660.
- Hogarth, W.H.J.; Muir, S.S.; Whittaker, A.K.; Diniz da Costa, J.C.; Drennan, J.; Lu, G.Q. Proton conduction mechanism and the stability of sol–gel titanium phosphates. Solid State Ion. 2007, 177, 3389–3394.
- Alberti, G.; Costantino, U.; Casciola, M.; Ferroni, S.; Massinelli, L.; Staiti, P. Preparation, characterization and proton con-ductivity of titanium phosphate sulfophenylphosphonate. Solid State Ion. 2001, 145, 249–255.
- Zhang, L.; Liu, X.; Sun, X.; Jian, J.; Li, G.; Yuan, H. Proton conduction in organically templated 3D open-framework vana-dium−nickel pyrophosphate. Inorg. Chem. 2019, 58, 4394–4398.
- Botez, C.E.; Tackett, R.J.; Hermosillo, J.D.; Zhang, J.; Zhao, Y.; Wang, L. High pressure synchrotron x-ray diffraction studies of superprotonic transitions in phosphate solid acids. Solid State Ion. 2012, 213, 58–62.
- Baranov, A.I. Crystals with disordered hydrogen-bond networks and superprotonic conductivity. Review. Crystallogr. Rep. 2003, 48, 1012–1037.
- Bagryantseva, I.N.; Gaydamaka, A.A.; Ponomareva, V.G. Intermediate temperature proton electrolytes based on cesium dihydrogen phosphate and Butyral polymer. Ionics 2020, 26, 1813–1818.
- Dreßler, C.; Sebastiani, D. Effect of anion reorientation on proton mobility in the solid acids family CsHyXO4 (X = S, P, Se, y = 1, 2) from ab initio molecular dynamics simulations. Phys. Chem. Chem. Phys. 2020, 22, 10738–10752.
- Taninouchi, Y.K.; Uda, T.; Awakura, Y.; Ikeda, A.; Haile, S.M. Dehydration behavior of the superprotonic conductor CsH2PO4 at moderate temperatures: 230 to 260 °C. J. Mater. Chem. 2007, 17, 3182–3189.
- Mathur, L.; Kim, I.-H.; Bhardwaj, A.; Singh, B.; Park, J.-Y.; Song, S.-J. Structural and electrical properties of novel phosphate based composite electrolyte for low-temperature fuel cells. Composites Part B 2020, 202, 108405.
- Otomo, J.; Tamaki, T.; Nishida, S.; Wang, S.; Ogura, M.; Kobayashi, T.; Wen, C.-J.; Nagamoto, H.; Takahashi, H. Effect of water vapor on proton conduction of cesium dihydrogen phosphate and application to intermediate temperature fuel cells. J. Appl. Electrochem. 2005, 35, 865–870.
- Uda, T.; Haile, S.M. Thin-membrane solid-acid fuel cell. Electrochem. Solid State Lett. 2005, 8, A245–A246.
- Navarrete, L.; Andrio, A.; Escolástico, S.; Moya, S.; Compañ, V.; Serra, J.M. Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices. Membranes 2019, 9, 49.
- Ponomareva, V.G.; Bagryantseva, I.N.; Gaydamaka, A.A. Study of the Phase Composition and Electrotransport Properties of the Systems Based on Mono- and Disubstituted Phosphates of Cesium and Rubidium. Chem. Sustain. Dev. 2019, 27, 238–245.
- Martsinkevich, V.V.; Ponomareva, V.G. Double salts Cs1-xMxH2PO4 (M = Na, K, Rb) as proton conductors. Solid State Ion. 2012, 225, 236–240.
- Ponomareva, V.G.; Bagryantseva, I.N. Superprotonic CsH2PO4-CsHSO4 solid solutions. Inorg. Mater. 2012, 48, 187–194.
- Ponomareva, V.G.; Uvarov, N.F.; Lavrova, G.V.; Hairetdinov, E.F. Composite protonic solid electrolytes in the CsHSO4-SiO2 system. Solid State Ion. 1996, 90, 161–166.
- Ponomareva, V.G.; Shutova, E.S.; Matvienko, A.A. Conductivity of Proton Electrolytes Based on Cesium Hydrogen Sulfate Phosphate. Inorg. Mater. 2004, 40, 721–728.
- Otomo, J.; Minagawa, N.; Wen, C.-J.; Eguchi, K.; Takahashi, H. Protonic conduction of CsH2PO4 and its composite with silica in dry and humid atmospheres. Solid State Ion. 2003, 156, 357–369.
- Ponomareva, V.G.; Shutova, E.S. High-temperature behavior of CsH2PO4 and CsH2PO4-SiO2 composites. Solid State Ion. 2007, 178, 729–734.
- Singh, D.; Singh, J.; Kumar, P.; Veer, D.; Kumar, D.; Katiyar, R.S.; Kumar, A.; Kumar, A. The Influence of TiO2 on the Proton Conduction and Thermal Stability of CsH2PO4 Composite Electrolytes. S. Afr. J. Chem. Eng. 2021, 37, 227–236.
- Singh, D.; Kumar, P.; Singh, J.; Veer, D.; Kumar, A.; Katiyar, R.S. Structural, thermal and electrical properties of composites electrolytes (1−x) CsH2PO4/x ZrO2 (0 ≤ x ≤ 0.4) for fuel cell with advanced electrode. SN Appl. Sci. 2021, 3, 46.
- Veer, D.; Kumar, P.; Singh, D.; Kumar, D.; Katiyar, R.S. A synergistic approach to achieving high conduction and stability of CsH2PO4/NaH2PO4/ZrO2 composites for fuel cells. Mater. Adv. 2021, 3, 409–417.
- Aili, D.; Gao, Y.; Han, J.; Li, Q. Acid-base chemistry and proton conductivity of CsHSO4, CsH2PO4 and their mixtures with N-heterocycles. Solid State Ion. 2017, 306, 13–19.
- Bagryantseva, I.N.; Ponomareva, V.G.; Khusnutdinov, V.R. Intermediate temperature proton electrolytes based on cesium dihydrogen phosphate and poly(vinylidene fluoride-co-hexafluoropropylene). J. Mater. Sci. 2021, 56, 14196–14206.
- Ponomareva, V.G.; Bagryantseva, I.N.; Shutova, E.S. Hybrid systems based on nanodiamond and cesium dihydrogen phos-phate. Mater. Today 2020, 25, 521–524.
- Ponomareva, V.G.; Bagryantseva, I.N. Effect of the excess protons on the electrotansport, structural and thermodynamic properties of CsH2PO4. Solid State Ion. 2017, 304, 90–95.
- Ponomareva, V.G.; Lavrova, G.V. The influence of Cs2HPO4·H2O impurity on the proton conductivity and thermal proper-ties of CsH2PO4. Solid State Ion. 2019, 329, 90–94.
- Wang, M.; Luo, H.-B.; Liu, S.-X.; Zou, Y.; Tian, Z.-F.; Li, L.; Liu, J.-L.; Ren, X.-M. Water assisted high proton conductance in a highly thermally stable and superior water-stable open-framework cobalt phosphate. Dalton Trans. 2016, 45, 19466–19472.
- Umeyama, D.; Horike, S.; Inukai, M.; Itakura, T.; Kitagawa, S. Inherent Proton Conduction in a 2D Coordination Frame-work. J. Am. Chem. Soc. 2012, 134, 12780–12785.
- Inukai, M.; Horike, S.; Chen, W.; Umeyama, D.; Itakurad, T.; Kitagawa, S. Template-directed proton conduction pathways in a coordination framework. J. Mater. Chem. A. 2014, 2, 10404–10409.
- Zhao, H.-R.; Xue, C.; Li, C.-P.; Zhang, K.-M.; Luo, H.-B.; Liu, S.-X.; Ren, X.-M. A Two-Dimensional inorganic−organic hybrid solid of manganese(II) hydrogenophosphate showing high proton conductivity at room temperature. Inorg. Chem. 2016, 55, 8971–8975.
- Phang, W.J.; Lee, W.R.; Yoo, K.; Ryu, D.W.; Kim, B.S.; Hong, C.S. PH-dependent proton conducting behavior in a met-al-organic framework material. Angew. Chem. Int. Ed. 2014, 53, 8383–8387.
- Nagarkar, S.S.; Unni, S.M.; Sharma, A.; Kurungot, S.; Ghosh, S.K. Two-in-one: Inherent anhydrous and water-assisted high proton conduction in a 3D metal-organic framework. Angew. Chem. Int. Ed. 2014, 53, 2638–2642.
- Nguyen, N.T.T.; Furukawa, H.; Gándara, F.; Trickett, C.A.; Jeong, H.M.; Cordova, K.E.; Yaghi, O.M. Three-dimensional met-al-catecholate frameworks and their ultrahigh proton conductivity. J. Am. Chem. Soc. 2015, 137, 15394–15397.
- Horike, S.; Chen, W.; Itakura, T.; Inukai, M.; Umeyama, D.; Asakura, H.; Kitagawa, S. Order-to-disorder structural transfor-mation of a coordination polymer and its influence on proton conduction. Chem. Commun. 2014, 50, 10241–10243.
- Ma, N.; Kosasang, S.; Yoshida, A.; Horike, S. Proton-conductive coordination polymer glass for solid-state anhydrous pro-ton batteries. Chem. Sci. 2021, 12, 5818–5824.
- Matsuda, Y.; Yonemura, M.; Koga, H.; Pitteloud, C.; Nagao, M.; Hirayama, M.; Kanno, R. Synthesis, crystal structure, and ionic conductivity of tunnel structure phosphates, RbMg1-xH2x(PO3)3y(H2O). J. Mater. Chem. A 2013, 1, 15544–15551.
- Antraptseva, N.M.; Solod, N.V.; Povshuk, V.A. Electrical conductivity of solid solution of Co(II)-Zn dihydrophosphate and its thermolysis products. Funct. Mater. 2015, 22, 322–326.
- Fan, D.; Tian, P.; Xu, S.T.; Wang, D.H.; Yang, Y.; Li, J.Z.; Wang, Q.Y.; Yang, M.; Liu, Z.M. SAPO-34 templated by dipropyla-mine and diisopropylamine: Synthesis and catalytic performance in the methanol to olefin (MTO) reaction. New J. Chem. 2016, 40, 4236–4244.
- Yu, Y.; Zhu, J.; Liu, J.; Yan, Y.; Song, X. Synthesis and characterization of two layered aluminophosphates [R-C8H12N]8[H2O]2·[Al8P12O48H4] and [S-C8H12N]8[H2O]2·[Al8P12O48H4] with a mirror symmetric feature and their proton conductivity. Dalton Trans. 2017, 46, 9157–9162.
- Park, S.H.; Choi, W.; Choi, H.J.; Hong, S.B. Organic-Free Synthesis of Silicoaluminophosphate Molecular Sieves. Angew. Chem. Int. Ed. 2018, 57, 9413–9418.
- Wang, Y.Y.; Sun, Y.J., Mu, Y.; Zhang, C.Q.; Li, J.Y.; Yu, J.H. Organotemplate-free hydrothermal synthesis of an alumino-phosphate molecular sieve with AEN zeotype topology and properties of its derivatives. Chem. Commun. 2014, 50, 15400–15403.
- Sun, Y.J.; Yan, Y.; Wang, Y.Y.; Li, Y.; Li, J.Y.; Yu, J.H. High proton conduction in a new alkali metal-templated open-framework aluminophosphate. Chem. Commun. 2015, 51, 9317–9319.
- Parise, J.B. Crystal Structures of Related Novel Aluminophosphate Frameworks: Aipo4-21(py), Aipo4-EN3 (en) and A Struc-tural Model for Alpo4-25. In Studies in Surface Science and Catalysis; Drzâj, S.H.B., Pejovnik, S., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; Volume 24, pp. 271–278.
- Parnham, E.R.; Morris, R.E. The ionothermal synthesis of cobalt aluminophosphate zeolite frameworks. J. Am. Chem. Soc. 2006, 128, 2204–2205.
- Wei, Y.; Tian, Z.; Gies, H.; Xu, R.; Ma, H.; Pei, R.; Zhang, W.; Xu, Y.; Wang, L.; Li, K.; et al. Ionothermal synthesis of an alu-minophosphate molecular sieve with 20-ring pore openings. Angew. Chem. Int. Ed. 2010, 49, 5367–5370.
- Liu, L.; Yang, J.; Li, J.; Dong, J.; Šišak, D.; Luzzatto, M.; McCusker, L.B. Ionothermal sythesis and structure analysis of an open-framework zirconium phosphate with a high CO2/CH4 adsorption ratio. Angew. Chem. Int. Ed. 2011, 50, 8139–8142.
- Nakayama, M.; Sugiura, Y.; Hayakawa, T.; Nogami, M. A novel proton conductor of imidazole–aluminium phosphate hy-brids in the solid state. Phys. Chem. Chem. Phys. 2011, 13, 9439–9444.
- Wang, K.; Li, T.; Zeng, H.; Zou, G.; Zhang, Q.; Zhien Lin, Z. Ionothermal synthesis of open-framework metal phosphates using a multifunctional ionic liquid. Inorg. Chem. 2018, 57, 8726–8729.
- Zhang, C.; Yan, Y.; Huang, Z.; Shi, H.; Zhang, C.; Cao, X.; Jiang, J. Triclinic AlPO-34 zeolite synthesized with nicotine and its proton conduction properties. Inorg. Chem. Commun. 2018, 96, 165–169.
- Xue, C.; Zou, Y.; Liu, S.-X.; Ren, X.-M.; Tian, Z.-F. Two different types of channels exhibiting distinct proton transport be-haviour in an open framework aluminophosphate. J. Solid State Chem. 2018, 258, 695–701.
- Mu, Y.; Wang, Y.Y.; Li, Y.; Li, J.Y.; Yu, J.H. Organotemplate-free synthesis of an open-framework magnesium alumino-phosphate with proton conduction properties. Chem. Commun. 2015, 51, 2149–2151.
- Fan, D.; Barrier, N.; Vicente, A.; Gilson, J.-P.; Clevers, S.; Dupray, V.; Coquereld, G.; Valtchev, V. Organic template-free syn-thesis of an open framework silicoaluminophosphate (SAPO) with high thermal stability and high ionic conductivity. Inorg. Chem. Front. 2020, 7, 542–553.
- Zhou, D.; Chen, L.; Yu, J.H.; Li, Y.; Yan, W.F.; Deng, F.; Xu, R.R. Synthesis, crystal structure, and solid-state NMR Spectros-copy of a new open-framework aluminophosphate (NH4)2Al4(PO4)4(HPO4)·H2O. Inorg. Chem. 2005, 44, 4391–4397.
- Zhao, H.R.; Jia, Y.; Gu, V.; He, F.Y.; Zhang, K.M.; Tian, Z.F.; Liu, J.L. A 3D open-framework iron hydrogenophosphate showing high proton conductance under water and aqua-ammonia vapor. RSC Adv. 2020, 10, 9046–9051.
- Zima, V.; Lii, K.-H. Synthesis and characterization of a novel one-dimensional iron phosphate: [C4H12N2]1.5[Fe2(OH)(H2PO4)(HPO4)2(PO4)]·0.5H2O. J. Chem. Soc. Dalton Trans. 1998, 24, 4109–4112.
- Lii, K.-H.; Huang, Y.-F. Large tunnels in the hydrothermally synthesized open-framework iron phosphate (NH3(CH2)3NH3)2[Fe4(OH)3(HPO4)2(PO4)3]·xH2O. Chem. Commun. 1997, 9, 839–840.
- Bazaga-García, M.; Colodrero, R.M.P.; Papadaki, M.; Garczarek, P.; Zon, J.; Olivera-Pastor, P.; Losilla, E.R.; Reina, L.L.; Aranda, M.A.; Choquesillo-Lazarte, D.; et al. Guest Molecule-Responsive Functional Calcium Phosphonate Frameworks for Tuned Proton Conductivity. J. Am. Chem. Soc. 2014, 136, 5731–5739.
- Lim, D.-W.; Kitagawa, H. Proton Transport in Metal−Organic Frameworks. Chem. Rev. 2020, 120, 8416–8467.
- Mamlouk, M.; Scott, K. A boron phosphate-phosphoric acid composite membrane for medium temperature proton ex-change membrane fuel cells. J. Power Sources 2015, 286, 290–298.
- Pusztai, P.; Haspel, H.; Tóth, I.Y.; Tombácz, E.; László, K.; Kukovecz, Á.; Kónya, Z. Structure-Independent Proton Transport in Cerium(III) Phosphate Nanowires. ACS Appl. Mater. Interfaces 2015, 7, 9947–9956.
- Petersen, H.; Stefmann, N.; Fischer, M.; Zibrowius, I.R.; Philippi, W.; Schmidt, W.; Weidenthaler, C. Crystal structures of two titanium phosphate-based proton conductors: Ab initio structure solution and materials properties. Inorg. Chem. 2022, 61, 2379–2390.
