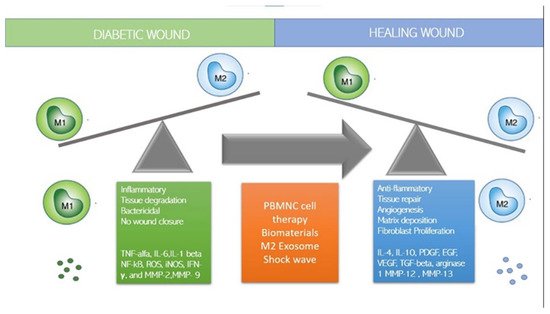
Cell-based therapies are rapidly emerging in regenerative medicine as dynamic treatments that perform multiple therapeutic functions. Monocytes and macrophages, as innate immune cells involved in inflammation control and tissue repair, are increasingly popular clinical candidates due to their robust angiogenic, anti-inflammatory, and regenerative ability.
Table 1 shows a brief description and clinical result of clinical trials based on macrophages or peripheral blood mononuclear cells describe in this review. The treatment of chronic ulcers with blood-derived macrophages activated by hypo-osmotic shock has been used effectively in over 1000 patients in Israel [
107]. Previously, Danon et al. in 1997 treated pressure ulcers in elderly patients by injecting macrophages from blood units of young, healthy donors near the wound periphery plus a portion of the cell suspension deposited on top of the wound [
113]. Patients were treated with a single implant, or with a second one when delayed healing was present 1 month later, and wound healing was compared with conventional methods (debridement, antibiotics, and wound dressings). In the macrophage-treated group, 27% healed, while only 6% healed in the control group (
p < 0.001). Moreover, the macrophage-treated group showed faster healing (
p < 0.02), and no side effects were reported [
113]. A second prospective controlled trial was designed to compare macrophage injections from healthy donors (66 patients) to standard care treatments (38 patients) for stage III and IV pressure ulcers in elderly patients. The results showed a significantly higher percentage of completely closed wounds in the macrophages-treated group in comparison to standard care [
114]. Interestingly, in the subset of diabetic patients, 65.5% of wounds with the macrophage treatment healed, while only 15.4% of healing was observed in the standard care group [
114]. Magenta et al. recently published an extensive review on autologous cell therapy from different tissue sources (blood, bone marrow, and adipose tissue) to treat critical limb ischemia in diabetic patients, reporting data from basic science to clinical trials [
115]. Autologous cell therapy, in particular, Peripheral Blood Mononuclear Cells (PBMNC), based on monocytes/macrophages and lymphocytes represent an interesting strategy to treat non-option critical limb patients and diabetic foot patients [
116,
117,
118,
119,
120,
121]. Rigato et al. on a recent meta-analysis on no-option critical limb ischemia (NO CLI) patients showed that PBMNCs, but not other cell types, were associated with a significant decrease in amputation and increase in amputation-free survival [
122]. The same results were observed by Liew et al. in a meta-analysis of 16 randomized trials where PBMNC lowered the risk of major amputation and increased ulcer healing significantly [
123]. Three other meta-analyses on autologous cellular therapy including PBMNC on diabetic foot patients showed a benefit of wound healing and reduced amputation associated with TcPO
2 increase and reduced pain [
124,
125,
126]. Dubsky et al. have treated 28 patients with diabetic foot disease (17 treated with bone marrow cells and 11 with PBMNC) comparing the result with a control group treated with standard care at 6 months and have reported a statistical increase in TcPO2 with no significant differences between bone marrow cells and peripheral blood cell groups, while no change in transcutaneous oxygen pressure in the control group was observed [
119]. In addition, the 6-month major amputation rate was significantly lower in the cell therapy group compared with that in the control group (11.1% vs. 50%), with no difference between bone marrow cells and peripheral blood cells [
119]. Interestingly, the same group reported a comparable improvement of CLI major amputation with autologous cell therapy in diabetic foot patients compared with repeated PTA and more effective healing of foot ulcers in the cell therapy group [
127]. A user-friendly point of care device based on peripheral blood selective filtration to be used for intra-operatory use in human cell therapy has been developed to produce fresh autologous PBMNC, with evidence in terms of adequate potency in therapeutic angiogenesis in vitro and in vivo [
128]. Promising results have been obtained from implanting PBMNC produced by a specific device (Hematrate Blood Filtration system Cook Regentec) in different clinical trials including diabetic patients [
120,
121,
129,
130]. Persiani et al. have observed a 9.4% decrease in major amputation in 18 no-option patients with diabetes treated by PBMNC together with an increase in TCPo2 and a pain reduction at 2 years [
120]. A similar result in terms of major amputation has also been previously reported on CLI non-option patients, including diabetic and Burgers patients, treated with PBMNC produced by apheresis [
116]. Interestingly, it has been demonstrated by a histological examination of incisional biopsies of diabetic non-healing ulcers that autologous PBMNC implants produced by this selective filtration point of care and injected perilesional around diabetic non-healing wounds polarize M1 macrophages in M2. Moreover, the implantation of A-PBMNC promotes relevant changes in the overall molecular setting over time [
71]. The consequent cellular and biochemical adaptations favour the establishment of conditions similar to physiological ones that progressively support the regeneration of damaged tissues and finally wound healing measured as inhibition of HIF, NF-KB, and TNF-alpha, progressive polarization of M1 into M2, increase in VEGF, and newly formed capillaries [
71]. As the regenerative processes occur, an increase in the vascular network formation is clearly seen [
71]. These preliminary data confirm in the ability of fresh, naïve, autologous PBMNC to induce immunomodulation through macrophage polarization and that this results in complete wound healing in a diabetic ulcer. On the contrary, the delivery of macrophages polarized in vitro into M2a and M2c phenotypes and then injected into mouse wounds did not accelerate healing in wild type mice and delayed healing in diabetic mice [
131]. The same study also observed a delayed re-epithelialization and persistence of neutrophils and M2 macrophages in diabetic treated wounds 15 days post-injury, suggesting that the application of ex vivo generated M2 macrophages is not beneficial and contraindicated for cell therapy of skin wounds. It seems instead that to produce a positive clinical outcome in terms of wound healing, polarization should occur in the patients in the wounded tissue which send the right microenvironmental signals to PBMNC. The same groups showed that the implants of Matrigel supplemented with M2a and M2c macrophage subsets in a mice wound model showed an increased number of endothelial cells and tubular structures, while M1-enriched Matrigel did not, suggesting that macrophages polarized towards an M2 phenotype seem to have a higher angiogenic potential compared to other subsets [
132]. Accordingly, Di Pardo et al. also observed an increase in VEGF and laminin in the diabetic wound after PBMC implant [
71]. A similar result was observed for the first time by De Angelis et al. in no-option CLI patients, including a subset of diabetic patients, after PBMNC implant [
121]: histological data confirmed dermal granulation tissue and an increased number of monocytes (CD68+) and newly formed microvessels (CD31+). After the PBMNC treatment in the healed epidermis, the presence of the new vessels was observed, whereas dermal inflammation and monocyte infiltration were reduced. All these data suggest that autologous PBMNC represent a safe, consolidated effective therapy for critical limb ischemia and diabetic foot non-healing wounds. Considering the low invasiveness and the repeatability, PBMNC could represent the new frontier that will replace stem cell therapy.