It is generally believed that more than 700 Conus species have evolved during the last 50 million years. With the highest species abundance occurring in southeast Asia, most Conus can be found in the shallow waters of tropical and subtropical oceans. Conus is widely distributed in rocky shores, sandy beaches, coral reefs and intertidal waters, with depths reaching up to over 600 m. Nowadays, Conus species are generally overexploited, and some species are now endangered. Exploring these waters of potentially high species diversity could enrich our understanding of their population’s genetic structure and provide the missing pieces for clarifying Conus evolution. As the conotoxin compounds vary greatly throughout the growth stages and across geolocations within the same species, further investigation of these species-specific regional distribution differences may provide crucial insights for artificial breeding and harvesting specific bioactive compounds in the future.
- Conus
- feeding habit
- phylogenetic tree
- distribution map
- biomedical compounds
- COVID-19
1. Introduction
| Conotoxin Family | Definition | Gene Superfamily | Cysteine Framework |
|---|---|---|---|
| α (ALPHA) | Nicotinic acetylcholine receptor (nAChR) | A, B3, D, J, L, M, S | I, II, III, IV, VIII, XIV, XX, XXV |
| γ (GAMMA) | Neuronal pacemaker cation currents (inward cation current) | O1, O2 | VI/VII |
| δ (DELTA) | Voltage-gated Na Channel (agonist, delay inactivation) | O1 | VI/VII |
| ε (EPISILON) | Presynaptic calcium channels or G protein-coupled presynaptic receptor | T | V |
| I (IOTA) | Voltage-gated Na Channel (agonist, no delayed inactivation) | I1, M | III, XI |
| κ (KAPPA) | Voltage-gated K Channel (blocker) | A, I2, J, M, O1 | III, IV, VI/VII, XI, XIV |
| μ (MU) | Voltage-gated Na Channel (antagonist, blocker) | M, O1,T | III, IV, V, VI, VII |
| ρ (RHO) | Alpha 1 adrenoreceptor (GPCR) | A | I |
| σ (SIGMA) | Serotonin-gated ion channels (GPCR) | S | VIII |
| τ (TAU) | Somatostatin receptor | T | V |
| χ (CHI) | Neuronal noradrenaline transporter | T | X |
| ω (OMEGA) | Voltage-gated calcium channel | O1, O2 | VI/VII, XVI, XXVI |
2. Feeding Habits and Evolution Path
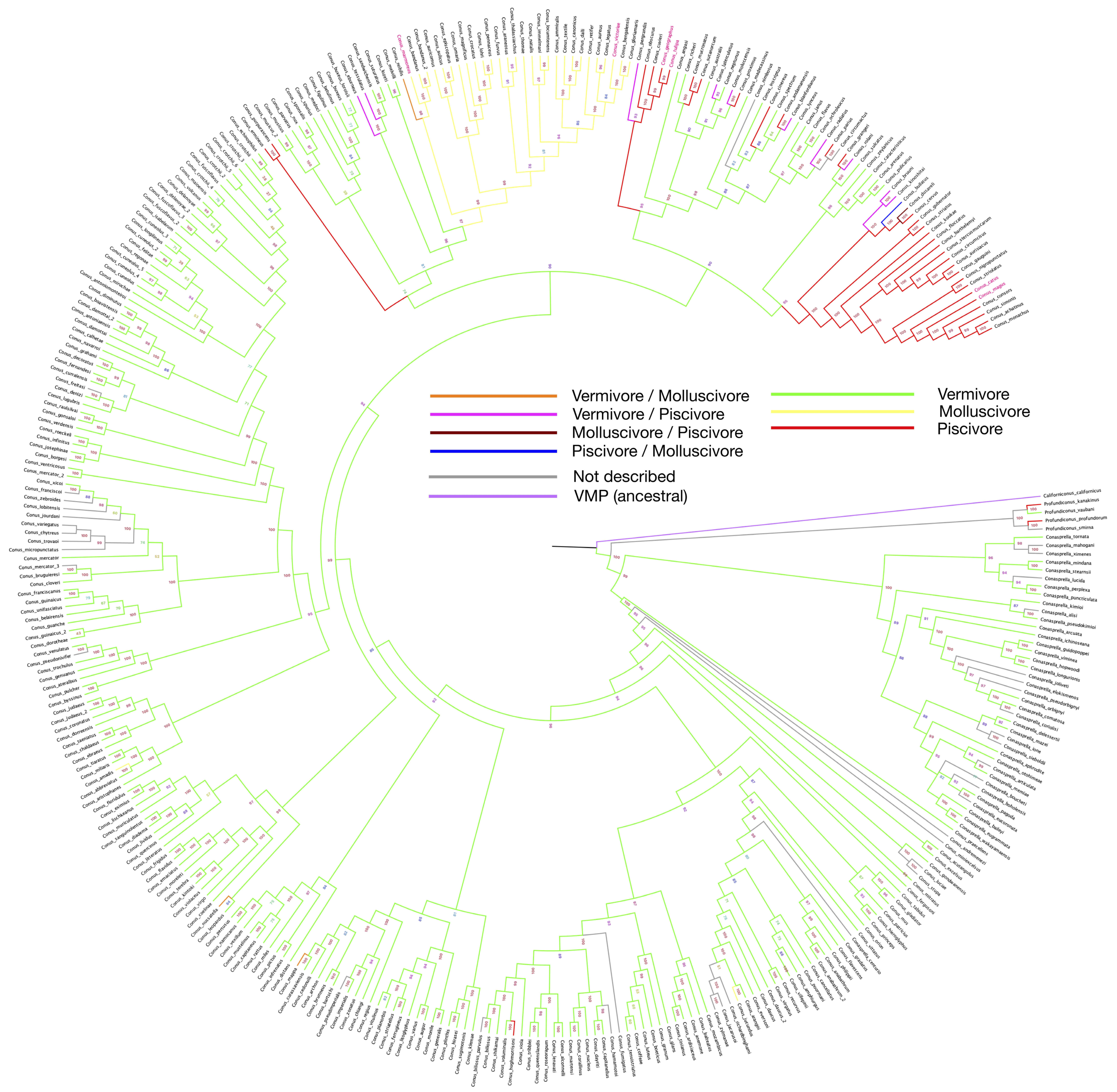
3. Distribution
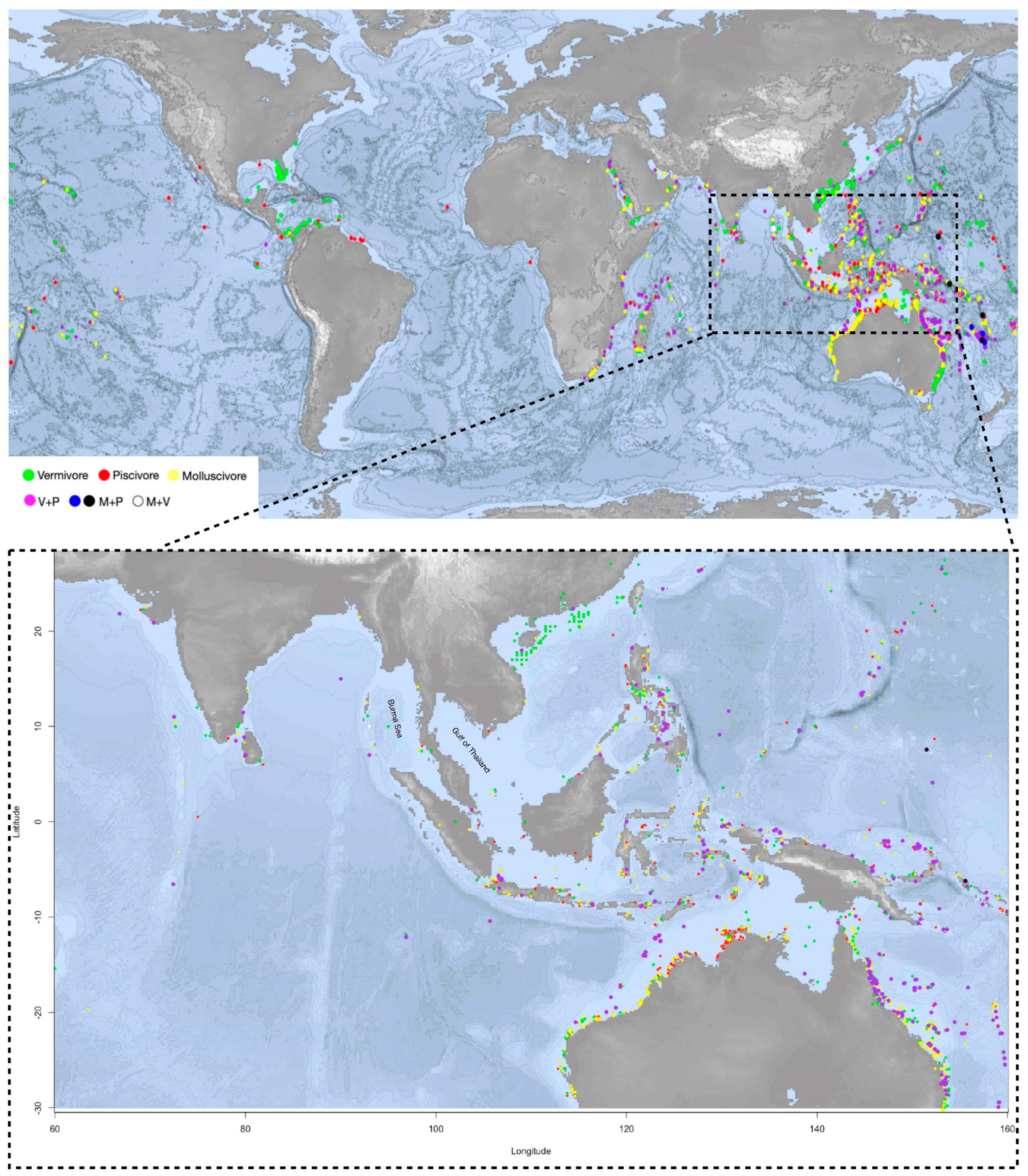
4. Conservation Status
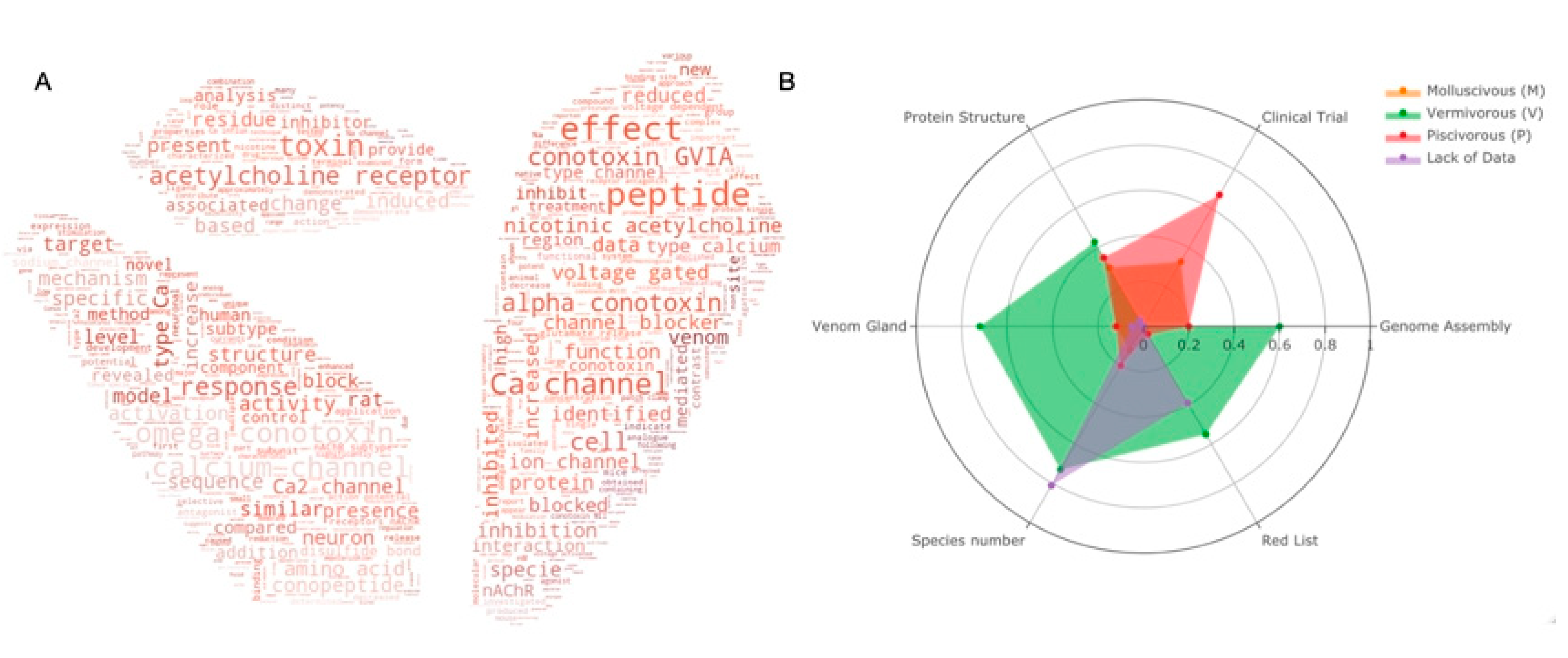
5. Drug Discovery
| Conopeptide | Commercial Name | Comment | Target | Stage | Company | Conus Species (**) | Reference | |
|---|---|---|---|---|---|---|---|---|
| 1 | α-Vc1.1 | ACV1 | Neuropathic pain | nAChR (α9α10) | Phase II * | Metabolic Pharmaceuticals, Melbourne, Australia | Conus victoriae (m) | [3] |
| 2 | ω-CVID | AM336 | Neuropathic pain | Ca2+ channel (CaV2.2) N-type calcium channels/blocker | Phase IIa * | Relevare Pharmaceuticals LTD., Australia | Conus catus (p) | [3] |
| 3 | μO-MrVIB | CGX-1002 | Neuropathic pain | Sodium channels/subtype selective blocker | Preclinical * | Cognetix Inc, Salt Lake City, USA | Conus tulipa (p) | [3] |
| 4 | Conantokin-G | CGX-1007 | Intractable epilepsy / pain | NMDA receptor (NR2B) | Preclinical * | Cognetix Inc, Salt Lake City, USA | Conus geographus (p) | [3] |
| 5 | Contulakin-G | CGX-1160 | Neuropathic pain | Neurotensin receptor | Phase Ib * | Cognetix Inc, Salt Lake City, USA | Conus geographus (p) | [3] |
| 6 | ω-MVIIA | SNX-III, C1002, Ziconotide, Prialt | Intractable pain | Ca2+ channel (CaV2.2) N-type calcium channels/blocker | FDA-approved | Elan Corporation (Elan Pharmaceuticals), CA, USA | Conus magus (p) | [3] |
| 7 | χ-MrIA | Xen2174 | Neuropathic pain | Norepinephrine transporter/inhibitor | Phase IIa * | Xenome, Ltd., Brisbane, Qld., Australia | n.a. | [3] |
| 8 | κ-PVIIA | CGX-1051 | Acute Myocardial Infarct, Cardioprotection | K+ channel (KV1)/blocker | Preclinical | n.a. | n.a. | [46] |
| 9 | n.a. | CGX-1204 | Muscle relaxer / pain | Nicotinic acetylcholine receptors/antagonist | Preclinical | n.a. | n.a. | [46] |
| 10 | μ-SIIIA | PEG-SIIIA | Inflammatory pain | Sodium channels/blocker | Preclinical | n.a. | n.a. | [46] |
| 11 | ρ-Conotoxin TIA | n.a. | n.a. | α-1 adrenergic receptors | Preclinical | Xenome, Ltd., Brisbane, Qld., Australia | Conus tulipa (p) | [47] |
| 12 | χ-conopeptides (χ-CTX MrIA/B) | n.a. | Neuropathic pain | Neurotransmitter transporters | Preclinical | Xenome, Ltd., Brisbane, Qld., Australia | Conus marmoreus (m) | [47] |
6. Structural and Functional Studies of Conotoxins with Their Receptors
7. Potential Value in COVID-19 and Other Diseases
This entry is adapted from the peer-reviewed paper 10.3390/md20020105
References
- Duda, T.F.; Kohn, A.J.; Palumbi, S.R. Origins of diverse feeding ecologies within Conus, a genus of venomous marine gastropods. Biol. J. Linn. Soc. 2001, 73, 391–409.
- Puillandre, N.; Duda, T.F.; Meyer, C.P.; Olivera, B.M.; Bouchet, P. One, four or 100 genera? A new classification of the cone snails. J. Molluscan Stud. 2015, 81, 1–23.
- Gao, B.; Peng, C.; Yang, J.; Yi, Y.; Zhang, J.; Shi, Q. Cone snails: A big store of conotoxins for novel drug discovery. Toxins 2017, 9, 397.
- Richard, G.; Rochelle, L. Panorama sur La Diversite des Conidae 110 Espèces Prédatrices des Plus Efficaces. 2021. Available online: https://www.researchgate.net/publication/353337774_PANORAMA_SUR_LA_DIVERSITE_DES_CONIDAE_110_especes_predatrices_des_plus_efficaces (accessed on 7 January 2022).
- Franklin, J.B.; Apte, D.A. Two New Records of Conidae (Mollusca: Caenogastropoda) from the Andaman Islands, India. J. Bombay Nat. Hist. Soc. 2020, 117, 152546.
- Franklin, J.B.; Fernando, A.A.; Chalke, B.A.; Krishnan, K.S. Radular morphology of Conus (Gastropoda: Caenogastropoda: Conidae) from India. Molluscan Res. 2007, 27, 111–122.
- Sanpanich, K.; Duangdee, T. A survey of marine mollusc diversity in the Southern Mergui Archipelago, Myanmar. Phuket Mar. Biol. Cent. Res. Bull. 2018, 75, 45–60.
- Lluisma, A.O.; Milash, B.A.; Moore, B.; Olivera, B.M.; Bandyopadhyay, P.K. Novel venom peptides from the cone snail Conus pulicarius discovered through next-generation sequencing of its venom duct transcriptome. Mar Genomics. Mar. Genom. 2012, 5, 43–51.
- Olivera, B.M. Conus venom peptides: Reflections from the biology of clades and species. Annu. Rev. Ecol. Syst. 2002, 33, 25–47.
- Jiménez-Tenorio, M. Cone radular anatomy as a proxy for phylogeny and for conotoxin diversity. In Proceedings of the CONODAYS—Satellite event to Natural Peptide to Drugs International, Zermatt, Switzerland, 7–9 December 2011.
- Leal, J.H.; Kohn, A.J.; Mensch, R. A Veliconcha Unveiled: Observations on the Larva and Radula of Conus spurius, with Implications for the Origin of Molluscivory in Conus. Am. Malacol. Bull. 2017, 35, 111–118.
- Dutertre, S.; Lewis, R.J. Cone Snail Biology, Bioprospecting and Conservation. In Snails: Biology, Ecology and Conservation; Nova Science Publishers: New York, NY, USA, 2013.
- Buczek, O.; Bulaj, G.; Olivera, B.M. Conotoxins and the posttranslational modification of secreted gene products. Cell. Mol. Life Sci. 2005, 62, 3067–3079.
- Neves, J.L.B.; Lin, Z.; Imperial, J.S.; Antunes, A.; Vasconcelos, V.; Olivera, B.M.; Schmidt, E.W. Small Molecules in the Cone Snail Arsenal. Org. Lett. 2015, 17, 4933–4935.
- Schulz, J.R.; Jan, I.; Sangha, G.; Azizi, E. The high speed radular prey strike of a fish-hunting cone snail. Curr. Biol. 2019, 29, R788–R789.
- Stewart, J.; Gilly, W.F. Piscivorous behavior of a temperate cone snail, Conus californicus. Biol. Bull. 2005, 209, 146–153.
- Dutertre, S.; Jin, A.-H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation-and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521.
- Abalde, S.; Jimenez-Tenorio, M.; Afonso, C.M.L.; Zardoya, R. Conotoxin diversity in Chelyconus ermineus (Born, 1778) and the convergent origin of piscivory in the atlantic and indo-pacific cones. Genome Biol. Evol. 2018, 10, 2643–2662.
- Prator, C.; Murayama, K.M.; Schulz, J.R. Venom variation during prey capture by the cone snail, Conus textile. PLoS ONE 2014, 9, e98991.
- Duda, T.F.; Chang, D.; Lewis, B.D.; Lee, T. Geographic variation in venom allelic composition and diets of the widespread predatory marine gastropod Conus ebraeus. PLoS ONE 2009, 4, e6245.
- Neves, J.; Campos, A.; Osório, H.; Antunes, A.; Vasconcelos, V. Conopeptides from Cape Verde Conus crotchii. Mar. Drugs 2013, 11, 2203–2215.
- Neves, J.L.B.; Imperial, J.S.; Morgenstern, D.; Ueberheide, B.; Gajewiak, J.; Antunes, A.; Robinson, S.D.; Espino, S.; Watkins, M.; Vasconcelos, V.; et al. Characterization of the First Conotoxin from Conus ateralbus, a Vermivorous Cone Snail from the Cabo Verde Archipelago. Mar. Drugs 2019, 17, 432.
- Mir, R.; Karim, S.; Kamal, M.A.; Wilson, C.; Mirza, Z. Conotoxins: Structure, Therapeutic Potential and Pharmacological Applications. Curr. Pharm. Des. 2016, 22, 582–589.
- Yuan, D.D.; Han, Y.H.; Wang, C.G.; Chi, C.W. From the identification of gene organization of α conotoxins to the cloning of novel toxins. Toxicon 2007, 49, 1135–1149.
- Kaas, Q.; Westermann, J.C.; Craik, D.J. Conopeptide characterization and classifications: An analysis using ConoServer. Toxicon 2010, 55, 1491–1509.
- Jin, A.H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.; Kaas, Q.; Craik, D.J.; Lewis, R.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem. Rev. 2019, 119, 11510–11549.
- Ekberg, J.; Craik, D.J.; Adams, D.J. Conotoxin modulation of voltage-gated sodium channels. Int. J. Biochem. Cell Biol. 2008, 40, 2363–2368.
- Kaas, Q.; Yu, R.; Jin, A.-H.; Dutertre, S.; Craik, D.J. ConoServer: Updated content, knowledge, and discovery tools in the conopeptide database. Nucleic Acids Res. 2011, 40, D325–D330.
- Schroeder, C.I.; Craik, D.J. Therapeutic potential of conopeptides. Future Med. Chem. 2012, 4, 1243–1255.
- Miljanich, G. Ziconotide: Neuronal Calcium Channel Blocker for Treating Severe Chronic Pain. Curr. Med. Chem. 2004, 11, 3029–3040.
- Herrick, C.J. The cranial and first spinal nerves of menidia: A contribution upon the nerve components of the bony fishes. Section 13. Conclusions. J. Comp. Neurol. 1899, 9, 419–455.
- Olivera, B.M. Conus peptides: Biodiversity-based discovery and exogenomics. J. Biol. Chem. 2006, 281, 31173–31177.
- Dutertre, S.; Jin, A.-H.; Kaas, Q.; Jones, A.; Alewood, P.; Lewis, R.J. Deep venomics reveals the mechanism for expanded peptide diversity in cone snail venom. Mol. Cell. Proteom. 2013, 12, 312–329.
- Duda, T.F.; Palumbi, S.R. Evolutionary diversification of multigene families: Allelic selection of toxins in predatory cone snails. Mol. Biol. Evol. 2000, 17, 1286–1293.
- Zhang, H.; Fu, Y.; Wang, L.; Liang, A.; Chen, S.; Xu, A. Identifying novel conopepetides from the venom ducts of Conus litteratus through integrating transcriptomics and proteomics. J. Proteom. 2019, 192, 346–357.
- Yao, G.; Peng, C.; Zhu, Y.; Fan, C.; Jiang, H.; Chen, J.; Cao, Y.; Shi, Q. High-throughput identification and analysis of novel conotoxins from three vermivorous cone snails by transcriptome sequencing. Mar. Drugs 2019, 17, 193.
- Li, Q.; Watkins, M.; Robinson, S.D.; Safavi-Hemami, H.; Yandell, M. Discovery of novel conotoxin candidates using machine learning. Toxins 2018, 10, 503.
- Abalde, S.; Tenorio, M.J.; Uribe, J.E.; Zardoya, R. Conidae phylogenomics and evolution. Zool. Scr. 2019, 48, 194–214.
- Puillandre, N.; Bouchet, P.; Duda, T.; Kauferstein, S.; Kohn, A.; Olivera, B.; Watkins, M.; Meyer, C. Molecular phylogeny and evolution of the cone snails (Gastropoda, Conoidea). Mol. Phylogenet. Evol. 2014, 78, 290–303.
- Krug, P.J. Patterns of speciation in marine gastropods: A review of the phylogenetic evidence for localized radiations in the sea. Am. Malacol. Bull. 2011, 29, 169–186.
- Uribe, J.E.; Puillandre, N.; Zardoya, R. Beyond Conus: Phylogenetic relationships of Conidae based on complete mitochondrial genomes. Mol. Phylogenet. Evol. 2017, 107, 142–151.
- Betancur, R.R.; Wiley, E.O.; Arratia, G.; Acero, A.; Bailly, N.; Miya, M.; Lecointre, G.; Ortí, G. Phylogenetic classification of bony fishes. BMC Evol. Biol. 2017, 17, 162.
- Kocot, K.M.; Cannon, J.; Todt, C.; Citarella, M.R.; Kohn, A.B.; Meyer, A.; Santos, S.R.; Schander, C.; Moroz, L.; Lieb, B.; et al. Phylogenomics reveals deep molluscan relationships. Nature 2011, 477, 452–456.
- Peters, H.; O’Leary, B.C.; Hawkins, J.P.; Carpenter, K.E.; Roberts, C.M. Conus: First comprehensive conservation red list assessment of a marine gastropod mollusc genus. PLoS ONE 2013, 8, e83353.
- Peters, H.; O’Leary, B.C.; Hawkins, J.P.; Roberts, C.M. The cone snails of Cape Verde: Marine endemism at a terrestrial scale. Glob. Ecol. Conserv. 2016, 7, 201–213.
- Han, T.S.; Teichert, R.W.; Olivera, B.M.; Bulaj, G. Conus Venoms—A Rich Source of Peptide-Based Therapeutics. Curr. Pharm. Des. 2008, 14, 2462–2479.
- Alonso, D.; Khalil, Z.; Satkunanthan, N.; Livett, B. Drugs From the Sea: Conotoxins as Drug Leads for Neuropathic Pain and Other Neurological Conditions. Mini Rev. Med. Chem. 2003, 3, 785–787.
- Pereira, F. Have marine natural product drug discovery efforts been productive and how can we improve their efficiency? Expert Opin. Drug Discov. 2019, 14, 717–722.
- Koh, D.C.I.; Armugam, A.; Jeyaseelan, K. Snake venom components and their applications in biomedicine. Cell. Mol. Life Sci. 2006, 63, 3030–3041.
- Lau, D.C.W.; Teoh, H. Benefits of Modest Weight Loss on the Management of Type 2 Diabetes Mellitus. Can. J. Diabetes 2013, 37, 128–134.
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997.
- Sunagar, K.; Fry, B.G.; Jackson, T.N.W.; Casewell, N.R.; Undheim, E.A.B.; Vidal, N.; Ali, S.A.; King, G.F.; Vasudevan, K.; Vasconcelos, V.; et al. Molecular evolution of vertebrate neurotrophins: Co-option of the highly conserved nerve growth factor gene into the advanced snake venom arsenal. PLoS ONE 2013, 8, e81827.
- Fry, B.G.; Undheim, E.; Ali, S.A.; Jackson, T.; Debono, J.; Scheib, H.; Ruder, T.; Morgenstern, D.; Cadwallader, L.; Whitehead, D.; et al. Squeezers and leaf-cutters: Differential diversification and degeneration of the venom system in toxicoferan reptiles. Mol. Cell. Proteom. 2013, 12, 1881–1899.
- Pineda, S.S.; Sollod, B.L.; Wilson, D.; Darling, A.; Sunagar, K.; Undheim, E.A.B.; Kely, L.; Antunes, A.; Fry, B.G.; King, G.F. Diversification of a single ancestral gene into a successful toxin superfamily in highly venomous Australian funnel-web spiders. BMC Genom. 2014, 15, 177.
- Ali, M. Studies on Bee Venom and Its Medical Uses. Int. J. Adv. Res. Technol. 2012, 1, 69–83.
- Guo, M.; Yu, J.; Zhu, X.; Zhangsun, D.; Luo, S. Characterization of an α 4/7-Conotoxin LvIF from Conus lividus That Selectively Blocks α3β2 Nicotinic Acetylcholine Receptor. Mar. Drugs 2021, 19, 398.
- Vincler, M.; Wittenauer, S.; Parker, R.; Ellison, M.; Olivera, B.M.; McIntosh, J.M. Molecular mechanism for analgesia involving specific antagonism of α9α10 nicotinic acetylcholine receptors. Proc. Natl. Acad. Sci. USA 2006, 103, 17880–17884.
- Castro, J.; Harrington, A.M.; Garcia-Caraballo, S.; Maddern, J.; Grundy, L.; Zhang, J.; Page, G.; Miller, P.E.; Craik, D.J.; Adams, D.J.; et al. α-Conotoxin Vc1.1 inhibits human dorsal root ganglion neuroexcitability and mouse colonic nociception via GABA B receptors. Gut 2016, 66, 1083–1094.
- Giribaldi, J.; Dutertre, S. α-Conotoxins to explore the molecular, physiological and pathophysiological functions of neuronal nicotinic acetylcholine receptors. Neurosci. Lett. 2018, 679, 24–34.
- Durek, T.; Craik, D.J. Therapeutic conotoxins: A US patent literature survey. Expert Opin. Ther. Patients 2015, 25, 1159–1173.
- Wang, H.; Li, X.; Zhangsun, D.; Yu, G.; Su, R.; Luo, S. The α9α10 nicotinic acetylcholine receptor antagonist αo-conotoxin GeXIVA alleviates and reverses chemotherapy-induced neuropathic pain. Mar. Drugs 2019, 17, 265.
- Zhu, X.; Pan, S.; Xu, M.; Zhang, L.; Yu, J.; Yu, J.; Wu, Y.; Fan, Y.; Li, H.; Kasheverov, I.E.; et al. High Selectivity of an α-Conotoxin LvIA Analogue for α3β2 Nicotinic Acetylcholine Receptors Is Mediated by β2 Functionally Important Residues. J. Med. Chem. 2020, 63, 13656–13668.
- Dyachenko, I.A.; Palikova, Y.A.; Palikov, V.A.; Korolkova, Y.V.; Kazakov, V.A.; Egorova, N.S.; Garifulina, A.I.; Utkin, Y.N.; Tsetlin, V.I.; Kryukova, E.V. α-Conotoxin RgIA and oligoarginine R8 in the mice model alleviate long-term oxaliplatin induced neuropathy. Biochimie 2021, in press.
- Kryukova, E.V.; Ivanov, I.A.; Lebedev, D.S.; Spirova, E.N.; Egorova, N.S.; Zouridakis, M.; Kasheverov, I.E.; Tzartos, S.J.; Tsetlin, V.I. Orthosteric and/or allosteric binding of a-conotoxins to nicotinic acetylcholine receptors and their models. Mar. Drugs 2018, 16, 460.
- Padilla, A.; Dovell, S.; Chesnokov, O.; Hoggard, M.; Oleinikov, A.V.; Marí, F. Conus venom fractions inhibit the adhesion of Plasmodium falciparum erythrocyte membrane protein 1 domains to the host vascular receptors. J. Proteom. 2021, 234, 104083.
- Brogna, C.; Cristoni, S.; Petrillo, M.; Querci, M.; Piazza, O.; Van den Eede, G. Toxin-like peptides in plasma, urine and faecal samples from COVID-19 patients. F1000Research 2021, 10, 550.
- Oliveira, A.S.F.; Ibarra, A.A.; Bermudez, I.; Casalino, L.; Gaieb, Z.; Shoemark, D.K.; Gallagher, T.; Sessions, R.B.; Amaro, R.E.; Mulholland, A.J.; et al. A potential interaction between the SARS-CoV-2 spike protein and nicotinic acetylcholine receptors. Biophys. J. 2021, 120, 983–993.
