Anthocyanins are considered high added-value compounds due to their sensory qualities, colors, and nutritional properties; they are considered bioactive ingredients. They are found in high concentrations in many by-products across the food industry. At present, expensive raw materials and production technologies make the extraction of natural anthocyanins relatively expensive.
- anthocyanins
- non-conventional extraction techniques
- anthocyanin yields
- industrial application
1. Introduction
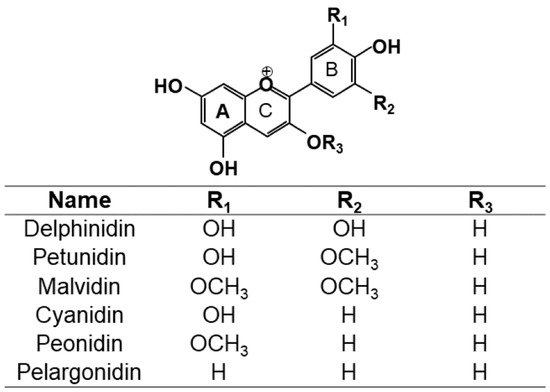
2. Extraction Methods Used to Extract Anthocyanins
2.1. Conventional Extraction Techniques
2.2. Non-Conventional Extraction Techniques
3. Advantages and Disadvantages of the Promising Green Extraction Techniques
|
Technique |
Strengths |
Weaknesses |
Suitability |
|---|---|---|---|
|
UAE |
Versatile, flexible, low cost, and very easy to use; fast energy transfers; low solvent usage; extraction time (5–60 min); can be combined with heating to improve the yield or with enzymatic treatment to improve the anthocyanin yield and the bioactivity of the extract; available on a large scale. |
Lack of homogeneity in the process improved by probe system (PUE); the large-scale application could be limited by the higher cost and nonlinearity of process; after the extraction, a filtration and clean-up step is required; the process can lead to operator fatigue. |
|
|
MAE |
Quick and homogeneous heating; low solvent usage; extraction time (1–40 min); currently, vacuum microwave extraction has been developed to provide a MAE method with a lower reactor temperature; possible application on a large scale. |
The solvent must absorb microwaves; the heating could damage the structure and the activity of some compounds; after the extraction, a filtration and clean-up step is required. |
|
|
SFE |
CO2 as a solvent; easy to remove after extraction; reduced the thermal degradation. Extraction time (up to 1 h); it does not require an alternative energy source; it is available on a large scale. |
Needs a co-solvent to extract polar compounds. The amount and type of co-solvent need to be optimize together with other parameters. SWE present the limitation of need high temperature to reach the subcritical condition, ethanol could be used instead of water. |
|
|
PLE |
Low solvent consumption; protection for oxygen and light sensitive compounds; it needs temperature; possible application on a large scale. |
Expensive equipment required; after the extraction, a clean-up step is required; extraction time (1–2 h). |
|
|
HHPE |
Short extraction time (~ 5 min); performed at room temperature; higher repeatability; smaller amount of solvents; possible application at large scale. |
High investment cost and cost maintenance and service; high pressure could affect the structure or activity of some compounds. The parameter should be optimized to avoid it. |
|
|
PEFE |
Short extraction time (less than 1 s); performed at room temperature; low energy and monetary costs; possible application on a large scale. |
Some compounds could be affected by high electric fields; it is desirable to reduce the electrical conductivity of the matrix before the extraction. For industrial application there are some problems related to: non-uniform distribution of the electric pulses, the suitable solvents are very limited and cooling system is necessary to control the temperature when extracting thermolabile compounds if high electrical pulses are applied. |
|
|
HVED |
Low temperature; short extraction time and energy input; possible application on a large scale. |
High cost maintenance and service; high voltage electrical discharges may generate chemical products and free reactive radicals, which can react with antioxidant compounds decreasing their bioactive activity. |
|
|
EAE |
Moderate extraction conditions; eco-friendly; selectivity due to the specificity of enzymes; can be combined with ultrasonic extraction to improve the yield and the bioactivity of the extract. |
Expensive cost of enzymes; activity of enzymes varying with the pH, temperature and nutrients of the matrix; after the extraction, a filtration and clean-up step is required. Difficulties to be applied on a large scale; extraction time (1–12 h); low availability of commercial enzyme types; sometimes they have low selectivity and variability. |
|
This entry is adapted from the peer-reviewed paper 10.3390/antiox11020286
References
- Jezek, M.; Zorb, C.; Merkt, N.; Geilfus, C.-M. Anthocyanin Management in Fruits by Fertilization. J. Agric. Food Chem. 2018, 66, 753–764.
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451.
- Dangles, O.; Fenger, J.A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970.
- Martín, J.; Kuskoski, E.M.; Navas, M.J.; Asuero, A.G. Antioxidant Capacity of Anthocyanin Pigments. In Flavonoids—From Biosynthesis to Human Health; Justino, J., Ed.; Science, Technology and Medicine Open Access Publisher: Rijeka, Croatia, 2017; Chapter 11; pp. 205–255.
- Martín Bueno, J.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jímenez, A.M.; Fett, R.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part II: Chemical structure, color, and intake of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151.
- Navas, M.J.; Jiménez-Moreno, A.M.; Martín Bueno, J.; Sáez-Plaza, P.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part IV: Extraction of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 313–342.
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2022, 88, 104864.
- Enaru, B.; Dretcanu, G.; Pop, T.D.; Stanila, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967.
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180.
- Morata, A.; Escott, C.; Loira, I.; López, C.; Palomero, F.; González, C. Emerging Non-Thermal Technologies for the Extraction of Grape Anthocyanins. Antioxidants 2021, 10, 1863.
- Albuquerque, B.R.; Pinela, J.; Barros, L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Anthocyanin-rich extract of jabuticaba epicarp as a natural colorant: Optimization of heat- and ultrasound-assisted extractions and application in a bakery product. Food Chem. 2020, 316, 126364.
- Fernandes, F.; Pereira, E.; Prieto, M.A.; Calhelha, R.C.; Ciric, A.; Sokovic, M.; Simal-Gandara, J.; Barros, L.; Ferreira, I.C.F.R. Optimization of the Extraction Process to Obtain a Colorant Ingredient from Leaves of Ocimum basilicum var. Purpurascens. Molecules 2019, 24, 686.
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259.
- Fincan, M. Potential Application of Pulsed Electric Fields for Improving Extraction of Plant Pigments. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer: Cham, Germany, 2017; Chapter 9; pp. 2171–2192.
- EFSA. Scientific Opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J. 2013, 11, 3145.
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537.
- Kumara, K.; Srivastava, S.; Sharanagatb, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochemistry 2021, 70, 105325.
- Gao, Y.; Ji, Y.; Wang, F.; Li, W.; Zhang, X.; Niu, Z.; Wang, Z. Optimization the extraction of anthocyanins from blueberry residue by dual-aqueous phase method and cell damage protection study. Food Sci. Biotechnol. 2021, 30, 1709–1719.
- Moirangthem, K.; Ramakrishna, P.; Amer, M.H.; Tucker, G.A. Bioactivity and anthocyanin content of microwave-assisted subcritical water extracts of Manipur black rice (Chakhao) bran and straw. Future Foods 2021, 3, 100030.
- Barba, F.J.; Grimi, N.; Vorobiev, E. New Approaches for the Use of Non-conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae. Food Eng. Rev. 2015, 7, 45–62.
- Carrera, C.; Aliaño-González, M.J.; Valaityte, M.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. A Novel Ultrasound-Assisted Extraction Method for the Analysis of Anthocyanins in Potatoes (Solanum tuberosum L.). Antioxidants 2021, 10, 1375.
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272.
- Jiang, H.; Yang, T.; Li, J.; Ye, H.; Liu, R.; Li, H.; Deng, Z. Response surface methodology for optimization of microwave-assisted extraction and antioxidant activity of anthocyanins from red rice. J. Chin. Inst. Food Sci. Technol. 2015, 15, 74–81.
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81.
- Perino-Issartier, S.; Zill-e-Huma; Abert-Vian, M.; Chemat, F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess Technol. 2011, 4, 1020–1028.
- Lončarić, A.; Jozinović, A.; Kovač, T.; Kojić, N.; Babić, J.; Šubarić, D. High Voltage Electrical Discharges and Ultrasound-Assisted Extraction of Phenolics from Indigenous Fungus-Resistant Grape By-Product. Pol. J. Food Nutr. Sci. 2020, 70, 101–111.
- Zhou, Y.; Zhao, X.; Huang, H. Effects of Pulsed Electric Fields on Anthocyanin Extraction Yield of Blueberry Processing By-Products. J. Food Process. Preserv. 2015, 39, 1898–1904.
- Varadharajan, V.; Shanmugam, S.; Ramaswamy, A. Model generation and process optimization of microwave assisted aqueous extraction of anthocyanins from grape juice waste. J. Food Process. Eng. 2017, 40, e12486.
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.I.; Bhatt, D.; Daglia, M.; Baldi, A.; Prasad Devkota, H.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102.
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149.
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20.
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436.
- Martín, J.; Asuero, A.G. High hydrostatic pressure for recovery of anthocyanins: Effects, performance, and applications. Sep. Purif. Rev. 2021, 50, 159–176.
- Xi, J. Ultrahigh pressure extraction of bioactive compounds from plants—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1097–1106.
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893.
- Paludo, M.; Colombo, R.; Teixeira, J.; Hermosín-Gutiérrez, I.; Ballus, C.; Godoy, H. Optimizing the Extraction of Anthocyanins from the Skin and Phenolic Compounds from the Seed of Jabuticaba Fruits (Myrciaria jabuticaba (Vell.) O. Berg) with Ternary Mixture Experimental Designs. J. Braz. Chem. Soc. 2019, 30, 1506–1514.
- Demirdöven, A.; Özdoğan, K.; Erdoğan-Tokatli, K. Extraction of Anthocyanins from Red Cabbage by Ultrasonic and Conventional Methods: Optimization and Evaluation. J. Food Biochem. 2015, 39, 491–500.
- Backes, E.; Pereira, C.; Barros, L.; Prieto, M.A.; Genena, A.K.; Barreiro, M.F.; Ferreira, I.C.F.R. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound based extraction techniques. Food Res. Int. 2018, 113, 197–209.
- Khazaei, K.M.; Jafari, S.M.; Ghorbani, M.; Kakhki, A.H.; Sarfarazi, M. Optimization of Anthocyanin Extraction from Saffron Petals with Response Surface Methodology. Food Anal. Methods 2016, 9, 1993–2001.
- Mane, S.; Bremner, D.H.; Tziboula-Clarke, A.; Lemos, M.A. Effect of ultrasound on the extraction of total anthocyanins from Purple Majesty potato. Ultrason. Sonochemistry 2015, 27, 509–514.
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 2015, 104, 122–131.
- Reategui, J.L.P.; da Fonseca Machado, A.P.; Barbero, G.F.; Rezende, C.A.; Martinez, J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids 2014, 94, 223–233.
- Andrade, T.A.; Hamerski, F.; Fetzer, D.E.L.; Roda-Serrat, M.C.; Corazza, M.L.; Norddahl, B.; Errico, M. Ultrasound-assisted pressurized liquid extraction of anthocyanins from Aronia melanocarpa pomace. Sep. Purif. Technol. 2021, 276, 119290.
- Faisal Manzoor, M.; Ahmed, Z.; Ahmad, N.; Karrar, E.; Rehman, A.; Aadil, R.M.; Al-Farga, A.; Waheed Iqbal, M.; Rahaman, A.; Zeng, X.A. Probing the combined impact of pulsed electric field and ultra-sonication on the quality of spinach juice. J. Food Process. Preserv. 2021, 45, e15475.
- Oancea, S.; Perju, M. Influence of enzymatic and ultrasonic extraction on phenolics content and antioxidant activity of Hibiscus Sabdariffa, L. flowers. Bulg. Chem. Commun. 2020, 52, 25–29.
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Ultrasound-Assisted Enzymatic Extraction of Anthocyanins from Raspberry Wine Residues: Process Optimization, Isolation, Purification, and Bioactivity Determination. Food Anal. Methods 2021, 14, 1369–1386.



