Type 1 diabetes (T1D) is an autoimmune disease characterized by the destruction of insulin-producing pancreatic β-cells by their own immune system, resulting in lifelong insulin deficiency. Continuous exogenous insulin replacement therapy is the current standard of care for T1D. Transplantation of primary pancreatic islets or the entire pancreas is a viable remedy for managing patients with autoimmune T1D. However, this strategy is not feasible due to several obstacles, including a scarcity of donors, islet cells, and poor vascular engraftment of islets post-transplantation, as well as the need for prolonged immune suppression.
1. Introduction
Autoimmune diseases, which are pathological conditions caused by abnormal immune responses to substances and tissues that are normally present or generated within their own body, affect nearly 23.5 million Americans, with nearly 80 percent of those affected being females. Around 100 or more autoimmune diseases have been reported, according to the American Autoimmune Related Diseases Association (AARDA). Many of them have similar symptoms, making them more difficult to diagnose and distinguish. Autoimmune diseases can affect almost any organ in the body, including the heart, muscles, eyes, brain, nerves, skin, joints, lungs, kidneys, glands, the digestive tract, and blood vessels. Although there are over 100 autoimmune diseases known to date, the most common autoimmune disorders are Type 1 Diabetes Mellitus (T1D), Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus (SLE), Inflammatory Bowel Disease (IBD), Multiple Sclerosis (MS), Guillain-Barre syndrome, chronic inflammatory demyelinating polyneuropathy, Psoriasis, Graves’ disease, and Hashimoto’s disease [
1,
2].
As we all know, one of the immune system’s most important functions is to protect the body by responding to attacking pathogens such as bacteria, parasites, or viruses by producing antibodies or sensitized lymphocytes to fight them. An immune response against one’s own body cannot be triggered or tolerated in healthy conditions. However, in some cases, the body’s immune cells or tissue make a mistake and behave as if they are foreign because our immune system recognizes them as foreign entities and attacks their own body rather than protecting them. These irrational responses can result in a variety of autoimmune diseases. T1D, also known as Juvenile Diabetes, is a chronic autoimmune disease in which both T and B lymphocytes initiate a specific targeted immune response that leads to the destruction of insulin-producing pancreatic β-cells [
3,
4,
5].
According to the Centers for Disease Control and Prevention, approximately 10% of the American population has diabetes, with type 1 diabetes accounting for 5–10% of those affected (T1D). The number of children diagnosed with various autoimmune diseases, in addition to autoimmune T1D, has increased. T1D is one of the most common childhood chronic diseases, affecting approximately 70,000 children each year. Worldwide, 111,1100 young people under the age of 20 have autoimmune T1D, and its prevalence in children is increasing by 3–5 percent per year, which is far too high. Pediatric T1D prevalence rates vary by region, but are typically lower in Asian countries and higher in the rest of the world [
6,
7]. Many autoimmune disorders, including T1D, are caused by flaws in the regulation of effector immune cell populations, which may be due to malfunctions in the immune-mediated suppression or immunological tolerance mechanisms.
T1D is a complex autoimmune disease characterized by genomic, epigenomic, and environmental factors that influence both adaptive and innate effector immune cell populations, culminating in pathological, chronic islet inflammation. The heterogeneity associated with human autoimmune T1D, as well as the nature of islet inflammation, reflects the individual’s genotype and type of environmental insult. These factors influence which immune effectors are important in the pathophysiology of autoimmune T1D, the rate of disease progression, and the degree of pancreatic islet-specific β-cell dysregulation and/or death [
8,
9,
10,
11].
The emergence of autoimmune disorders resulted from the immune system’s inability to control autoreactive cascading responses. The ability to reprogram the immune system to maintain homeostasis without ongoing treatment is the holy grail of autoimmune therapies such as T1D. The current treatment regimen for T1D patients is largely based on exogenous insulin injections or insulin pumps, which are lifesaving but not curative. While insulin replacement is beneficial for improving glycol-metabolic control, it is limited by its inherent inability to replicate endogenous insulin’s biological functions; this puts T1D patients at risk for hypoglycemic episodes [
12]. Due to the accelerated T1D disease, autoimmune T1D patients have a significantly reduced life expectancy. There are several ways to rebuild complete endocrine pancreatic function, but cadaveric whole pancreas or islets transplantation is only available to a small number of T1D patients. There was limited clinical success due to poor post-transplant engraftment of vascular cells, blood-mediated inflammatory response, hypoxia, hypoxia-reoxygenation injury, the alloimmune response against the graft, and persistent autoimmune attacks in T1D patients who received islets transplanted [
13,
14].
2. Background of Autoimmune T1D
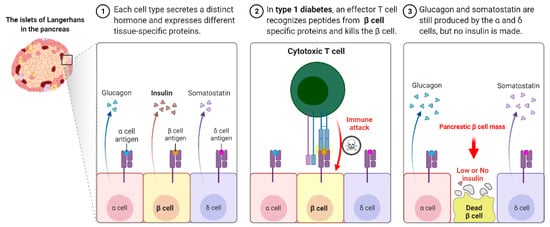
Autoimmune T1D occurs when our own immune system mistakenly destroys the insulin hormone-producing pancreatic β-cells in the Langerhans islets, schematically shown in Figure 1. T1D appears to have a genetic component and can be diagnosed in childhood and reported in later life, i.e., adulthood. Its causes are not completely understood or researched, and there is currently no likely treatment or cure. To survive, T1D patients must rely on injected or pumped insulin for the rest of their lives. T1D is found in both children and adults who exhibit symptoms such as frequent urination, dry mouth, increased thirst, itchy or dry skin, increased appetite, unexplained weight loss, and infections. Individuals with autoimmune T1D are typically diagnosed after exhibiting symptoms such as nausea, vomiting, extreme thirst, exhaustion, and/or malaise. As the body loses its ability to produce insulin, a hormone that allows the body to use the sugar found in everyday foods, known as glucose, as an energy source, patients with T1D must work closely with their endocrinologists to determine the insulin doses and lifestyle changes needed to manage their blood sugar levels. Autoimmune T1D patients are vulnerable to a variety of health issues, ranging from minor to severe; if you are not properly managed, your glucose levels will rise. Most T1D patients spend their lives managing it and have a problem with it, blood glucose levels are outside the clinically recommended beneficial range, which leads to potentially fatal hyperglycemia (high blood glucose) episodes and hypoglycemia (low blood glucose). Assume you do not maintain it and develop high blood sugar, which frequently leads to devastating health complications later in life, such as blindness, kidney failure, heart disease, and nerve damage, resulting in amputations.
Figure 1. Type I Diabetes immune response.
Several factors are responsible for the causes of T1D; the scientific community does not distinguish between the exact causes of autoimmune T1D, but they do see some onset factors and triggers associated with the condition. There is frequently a family history of T1D, and the best diagnoses occur in people who have no family members with the disease. Having a family history of autoimmune T1D, on the other hand, puts people at an increased risk of developing the disease. Some scientists believe viral infections can cause or contribute to the onset of the disease. To clarify, developing a viral-based vaccine may be one method of preventing T1D. Some environmental factors may be linked to T1D, most likely because of changes in our environment, possibly via a few viral infections or other similar agents. Early exposure to those factors after birth may be linked to the development of autoimmune T1D. T1D is not caused by diet or lifestyle, but several studies point to genetics [
15,
16,
17,
18], ethnicity, age, and the likelihood of developing T1D; the presence of specific genes appears in many autoimmune T1D patients. As T1D does not discriminate, if someone in your family has had autoimmune T1D, you are more likely to develop the disease. Assume that people of all ethnic backgrounds have T1D, even though the prevalence increases in populations north of the equator. However, an individual can develop autoimmune T1D at any age, with the majority of cases being diagnosed in early elementary school or as preteens. Hormonal changes, like those associated with growth spurts, can have an impact on the presentation and management of T1D.
2.1. Immune Cells Involved in the Pathophysiology of Autoimmune Type 1 Diabetes
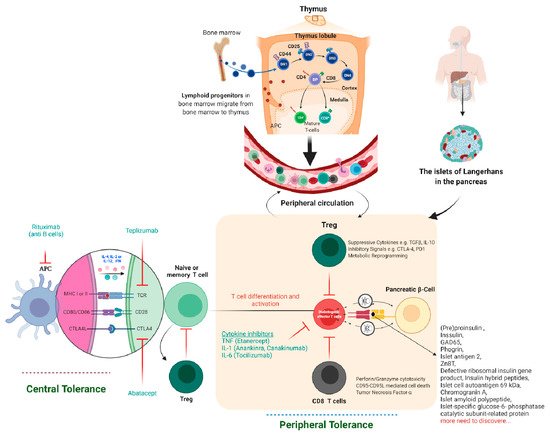
Tolerance has two mechanisms: central and peripheral tolerance. Both contribute to the defense against autoimmunity (Figure 2). T1D autoimmune disease can be caused by critical insufficiencies or restrictions in peripheral (lymph nodes/circulation) and/or central tolerance mechanisms (bone marrow/thymus), presenting a therapeutic opportunity. In healthy people, our immune systems are perfectly balanced to distinguish which antigens are foreign and which are self-antigens. To control this process, our immune system has a tolerance mechanism that includes thymic (central) tolerance, which eliminates high-affinity auto-antigen-specific T lymphocytes and those that fail to recognize auto-antigens completely and excuses T lymphocytes that recognize auto-antigens with intermediate affinity. As the naive immune repertoire is positively selected on autoantigens, self-recognition is hard-wired in the immune system, blurring the distinction between unwanted autoimmunity and desired immunity. As recirculating lymphocytes are exposed to tissue antigens under non-inflammatory conditions, which result in a tolerant, anergic state, our immune system’s peripheral tolerance system usually keeps potentially auto-specific lymphocytes in check. Nonetheless, in the presence of danger signals, such as infection and tissue damage, self-tolerance can be broken down, and autoimmune disease can progress. The immune pathogenesis of autoimmune T1D begins with a breakdown in self-tolerance, which results in the destruction of pancreatic β-cells by T lymphocytes (Figure 2).
Figure 2. Immune tolerance, therapeutics, and pancreatic β-cell-specific antigen discovered till now in T1D. The lymphoid progenitor cells are initially generated in the host bone marrow by special hematopoietic stem cells. The T lymphocytes travel to the thymus, where the thymus-based central tolerance mechanisms train T cells to discriminate between self and non-self (adverse selection). CD4 regulatory T cells (Tregs) and diabetogenic T lymphocytes may identify self or pancreatic β-cell-specific antigens, but at different affinities, which might explain their destructive actions β-cell. Subsequently, live T lymphocytes arrive in the blood and lymph nodes’ peripheral circulatory system and clash with their specific peptide-MHC/HLA complex. Specifically, in autoimmune T1D, these T lymphocytes are specific for pancreatic β-cell proteins such as insulin, GAD55, or so many others discovered to date. If these pancreatic β-cell-specific T lymphocytes and their respective antigen/epitopes are displayed by the MHC/HLA of antigen-presenting cells (DC, Macrophage, or B cells), T lymphocytes will become initiated in the lymph node, migrate to the pancreatic islets, and begin the demolition of β-cell in an antigen-specific manner. Tregs characterize the suppressive lymphocytes mainly responsible for peripheral immunological tolerance and try to inhibit these events. If the host body is unable to stop the autoimmune attack on pancreatic β-cells, insulin deficiency, hyperglycemia, and, eventually, autoimmune T1D will result. The majority of signaling events occur in the peripheral-local environment, in the lymph nodes and pancreas, and cannot be tracked using biomarkers.
Similarly, unwanted autoimmunity and desired host anti-pathogen specific immunity are inextricably linked, as effector immune responses that affect inflammatory tissue damage are analogous to those that mediate effective host defense. As a result, immunotherapeutic regimens that target the immune system’s common pathways invariably have both desired and unintended consequences. Several strategies for eliminating auto-antigen-specific immune cells by breaking tolerance to self-antigens can result in autoimmunity, which has been thoroughly reviewed [
19]. Recent findings emphasize the importance of inhibitory receptors (IRs), which trigger or regulate autoimmunity’s peripheral tolerance. Later, deletion and blockade studies in animals and humans show a link to positive disease outcomes, highlighting the potential clinical benefits of enhancing IR signaling, specifically CTLA4, PD1, LAG3, TIM3, and TIGIT, to treat autoimmune diseases.
2.2. Immunotherapy-Based Approaches to Treating Autoimmune T1D
Drugs that target immune system elements provide relief for millions of people suffering from autoimmune diseases such as psoriasis and rheumatoid arthritis. Still, no single immunotherapy-based treatment for autoimmune T1D is currently approved. Few research organizations and pharmaceutical companies focus on multiple aspects of the immune system to develop an effective immune cell-based therapy to cure autoimmune T1D. Individuals with autoimmune T1D require lifetime medications in the form of regular insulin prescriptions to manage the condition, putting them at high risk of long-term complications. One day, the immunotherapy-based strategy will benefit T1D and become an insulin-free substitute to stop, prevent, and possibly cure this autoimmune T1D. They have focused on immune cell-based approaches for autoimmune T1D treatment since the 1980s, considering several possibilities such as the repair of unblanched immune tolerance, inhibition of diabetogenic T-cell or B-cell functionality, ex vivo regulatory T-cell (Treg) generation, repression of the innate arm of the immune system, inflammation, and HLA-matched islet transplantation rejection.
2.3. There Are a Few Obstacles to Immunotherapy-Based Treatment for Autoimmune T1D Cure
First, thanks to the boon of recombinant technology, insulin has long been available, so there has not been a pressing need for the development of new T1D therapies. T1D patients must check their blood sugar levels regularly and calculate the amount of insulin to inject. Still, let us assume they can keep this up indefinitely. In that case, it is possible to live a fairly normal life. Second, the primary reason for the lack of approved immunotherapies for T1D is that clinicians have been hesitant to refer patients with T1D to immunotherapy-based clinical trials. Due to endocrinologists’ lack of understanding, new-generation immunotherapies have fewer unpleasant and potentially dangerous side effects than older immune-suppressing drugs or immunotherapy only for oncology, as it was initially explored or assumed. Third, there has been a lack of engagement from pharmaceutical companies and research organizations, and fourth, compared to a disease like RA, people living with T1D represent a relatively small business discussed [
20,
21,
22].
The first immunotherapy trials for T1D were conducted more than 35 years ago. No single study has demonstrated clinically significant benefits from therapy with an acceptable risk profile [
23]. A French Cyclosporine Diabetes Study was the first clinical trial to test the immunological intervention in type 1 diabetes [
24]. Cyclosporine A (CSA) disrupts TCR-mediated signal transduction, inhibiting T-cell activation and T helper IL-2 secretion [
25]. Later, a few studies demonstrated a significant reduction in exogenous insulin requirement after one year of immune-suppressive drug CSA treatment [
24,
26]. After CSA removal, blood glucose control worsens, and autoantibody levels are returned [
27].
Furthermore, cyclosporin medication has been linked to renal and pancreatic β-cell toxicity [
27]. Despite the lack of long-term effects and potential toxicity, these trials heralded a new clinical era centered on immunomodulatory strategies to delay or prevent T1D. Numerous interventions, including parenteral insulin administration, dietary exposures, broad-spectrum immunosuppressants, anti-inflammatory drugs, and T- or B-cell targeted immunosuppressants, have been tested to date. At the same time, a small number of clinical trials have revealed modest benefits. There are no clinically approved immunotherapies for autoimmune T1D now, but a few developments are listed in
Table 1 or highlighted in
Figure 2. Let us look at a few of these immunotherapies and how they might help T1D patients.
Table 1. Representative Immune-based therapeutic intervention study in autoimmune T1D and outcomes.
|
Therapeutic Agents
|
Study/Authors and Intervention
|
Outcome
|
Citations
|
|
T cell-based:
-
Anti-CD3 antibodies
-
Otelexizumab/teplizumab
-
prevent activation of T-cells,
-
deplete Teffs, and restore the Teffs/Tregs ratio
|
DEFEND-1, 2 (Otelexizumab)
|
There was no EBV in the therapy group, but there was no statistically significant difference in 2-h MMTT AUC C-peptide at 12 months.
|
[28]
|
|
Protégé (Teplizumab)
|
At 1 year, there was no significant difference in HbA1c1 < 6.5 percent or insulin dose < 0.5 U/kg per day: At year 2, AUC C-peptide in the high dose group was considerably greater than in the placebo group.
|
[29,30]
|
|
AbATE (Teplizumab)
|
The treatment group’s baseline adjusted AUC C-peptide reduced at year 2 was considerably lower.
|
[31]
|
|
B cell-based:
The monoclonal anti-CD20 antibody, which blocks the B cell function
|
Rituximab
|
HbA1c lowers as the rate of C peptide declines and insulin levels decrease.
|
[32,33]
|
|
Co-stimulation blockade
|
TrialNet CTLA4-Ig (abatacept); CTLA-4-IgG1 chimeric protein acts as a decoy receptor for CD80/86 and blocks CD28-CD80/86 induced co-stimulation of T-cells
|
Significantly higher stimulated C-peptide 2-h AUC in the treated group at the end of treatment and 1-year post-treatment
|
[34,35]
|
|
TIDAL (alafacept); Alafacept: chimeric protein (2 LFA-3 molecule-IgG1) binds to CD2 and blocks T-cell-stimulation
|
Significantly higher stimulated AUC C-peptide in the treatment group
compared to placebo; insulin use lower in the treatment group
|
[36]
|
|
Cytokine-based:
IL-2 agonist
|
Aldesleukin; IL-2 maintains Treg population and function
|
A dose-dependent elevation of Treg cells in the treatment group compared to placebo
|
[37]
|
|
TNF antagonism
|
Etanercept
|
HbA1c decreases while endogenous insulin production increases.
|
[38]
|
|
IL-1 receptor blockade
|
Anakinra
|
|
[39,40]
|
|
IL-1beta antagonism
|
Canakinumab
|
There was no C peptide reaction
|
[39]
|
|
IL-1 receptor blockade IL-1beta antagonism
|
Anakinra/canakinumab
|
Immunomodulation/reverse relationship between inflammation and C peptide stimulation
|
[41]
|
|
Anti-IL-6 therapy
|
Tocilizumab in New-onset T1D (EXTEND)
|
Ongoing study
|
Clinical trial NCT02293837
|
|
Antigen-based therapy:
|
Antigen-specific therapies may involve direct targeting of pathogenic T cells and/or boosting Tregs for bystander suppression
|
Tregs were shown to be more prevalent in those who got a larger dose of oral insulin (62.5 mg)
|
[42]
|
|
Treg-based:
|
Expansion of autologous Treg cells
|
A subset of adoptively transferred Treg is still in circulation (25% of peak) at year 1, with no significant adverse effects. C-peptide preservation in those receiving a lower dose
|
[20]
|
|
DC-based:
|
In T1D individuals who get their autologous DCs exhibited limited output. In this study, autologous DCs were given by infused via abdominal intradermal injections each 2 weeks apart
|
The autologous DC-based therapy was very well tolerated; no important differences were seen in glycemia
|
[37]
|
|
Combination therapy
|
|
C-peptide significantly increased at 30 months follow up; increased side effects
|
[43]
|
|
32% were insulin-free at 4 years, maintenance of C-peptide, but with increased side effects
|
[44]
|
|
Mean AUC C-peptide at 12 months was significantly higher in the study group compared to the placebo group
|
Low-dose ATG + plus pegylated G-CSF [45]
|
AbATE = Autoimmunity Blocking Antibody for Tolerance in Recently Diagnosed T1Ds, ATG = anti-thymocyte globulin, AUC = area under curve, CTLA-4 = cytotoxic T-lymphocyte-associated antigen, DEFEND = Durable Response Therapy Evaluation for Early or T1D = Type 1 Diabetes, EBV = Epstein Barr virus, G-CSF = granulocyte colony-stimulating factor, HbA1c = glycated haemoglobin, IL-2 = interleukin-2, LFA-3 = leukocyte function antigen-3, MMTT = mixed meal tolerance test, Pre-POINT = Primary intervention with Oral Insulin for Prevention of T1D in infants at high genetic risk, TCR = T-cell receptor, Teffs = T effector cells, TIDAL = Type 1 Diabetes with Alefacept, Tregs = T regulatory cells, DC = dendritic cells.
This entry is adapted from the peer-reviewed paper 10.3390/diabetology3010007