Fetal acidosis or intrauterine hypoxia refers to a condition of deprived short supply of oxygen below the physiologic level of normal blood, which is defined as pH ≤ 7.25.
- fetal acidosis
- fetal scalp
- magnetic induction
1. Introduction
Fetal blood sampling (FBS) is a well-established technique for monitoring fetal acidosis with high precision. However, due to its invasive nature, bleeding and infection are the primary concerns [1][2]. Several non-invasive techniques such as a Doppler ultrasound, tocodynamometer, cardiotocogram, fetal pulse oximetry, and fetal electrocardiograph have been suggested to address these concerns. However, the low accuracy and precision of these methods result in faulty analysis, impairing decision-making, as evidenced by the rising number of unnecessary C-sections in recent years [3][4] . Current non-invasive techniques are insufficient and require significant design enhancements to meet standard requirements. Additionally, alternative methods for providing minimally invasive and more continuous monitoring devices should be explored to fulfill the clinical needs of intrapartum fetal monitoring [1].
2. pH Measurement for Acidosis
| Measurands | Interpretation | |
|---|---|---|
| pH | Lactate | |
| ≥7.25 | ≤4.1 mmol/L | Normal |
| 7.21–7.24 | 4.2–4.8 mmol/L | Borderline |
| ≤7.20 | ≥4.9 mmol/L | Abnormal |
3. Current Fetal Acidosis Detection Method
3.1. Invasive Method
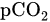 and base access. FBS is considered after CTG is pathological and considers expediting the birth [13]. FBS requires cervical dilation of 3–4 cm to accommodate a large amnioscope [14]. It is followed by a small scalpel incision to a depth of 1.5–2 mm [13] to obtain a blood sample using a thin heparinized capillary tube and transportation to the laboratory for analysis. Reduced blood pH serves as an early warning sign of acidosis, which is important for the obstetrician [15]. If the blood sample results are borderline (pH 7.1–7.24), a follow-up sample should be taken 30 min later, while birth should be expedited in cases of pH ≤ 7.20 [16].
and base access. FBS is considered after CTG is pathological and considers expediting the birth [13]. FBS requires cervical dilation of 3–4 cm to accommodate a large amnioscope [14]. It is followed by a small scalpel incision to a depth of 1.5–2 mm [13] to obtain a blood sample using a thin heparinized capillary tube and transportation to the laboratory for analysis. Reduced blood pH serves as an early warning sign of acidosis, which is important for the obstetrician [15]. If the blood sample results are borderline (pH 7.1–7.24), a follow-up sample should be taken 30 min later, while birth should be expedited in cases of pH ≤ 7.20 [16].3.2. Non-Invasive Method
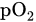 based on the relative absorbance of multiple light wavelengths by oxyhemoglobin and deoxyhemoglobin, which produces a periodic time domain signal called a photoplethysmogram (PPG). PPG pulse depth at two wavelengths is used to estimate the relative concentration of HbO2
based on the relative absorbance of multiple light wavelengths by oxyhemoglobin and deoxyhemoglobin, which produces a periodic time domain signal called a photoplethysmogram (PPG). PPG pulse depth at two wavelengths is used to estimate the relative concentration of HbO2This entry is adapted from the peer-reviewed paper 10.3390/s22041334
References
- Cummins, G.; Kremer, J.; Bernassau, A.; Brown, A.; Bridle, H.L.; Schulze, H.; Bachmann, T.T.; Crichton, M.; Denison, F.C.; Desmulliez, M.P.Y. Sensors for Fetal Hypoxia and Metabolic Acidosis: A Review. Sensors 2018, 18, 2648. [Google Scholar] [CrossRef]
- Demaegd, H.M.I.; Bauters, E.G.R.; Page, G.H. Foetal scalp blood sampling and ST-analysis of the foetal ECG for intrapartum foetal monitoring: A restricted systematic review. Facts Views Vis. ObGyn 2020, 11, 337–346. [Google Scholar]
- Carbonne, B.; Pons, K.; Maisonneuve, E. Foetal scalp blood sampling during labour for pH and lactate measurements. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 30, 62–67. [Google Scholar] [CrossRef]
- Opiyo, N.; Young, C.; Requejo, J.H.; Erdman, J.; Bales, S.; Betrán, A.P. Reducing unnecessary caesarean sections: Scoping review of financial and regulatory interventions. Reprod. Health 2020, 17, 133. [Google Scholar] [CrossRef]
- Popescu, M.R.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Peltecu, G.; Zagrean, A.-M. Getting an Early Start in Understanding Perinatal Asphyxia Impact on the Cardiovascular System. Front. Pediatr. 2020, 8, 68. [Google Scholar] [CrossRef]
- Zulkarnay, Z.; Shazwani, S.; Ibrahim, B.; Jurimah, A.J.; Ruzairi, A.R.; Zaridah, S. An overview on pH measurement technique and application in biomedical and industrial process. In Proceedings of the 2015 2nd International Conference on Biomedical Engineering (ICoBE), Penang, Malaysia, 30–31 March 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Factors That Contribute to Normal Labor-The Three Ps. Available online: https://www.brainkart.com/article/Factors-That-Contribute-to-Normal-Labor---The-Three-Ps_25661/ (accessed on 10 August 2021).
- Ayres-De-Campos, D. Acute Fetal Hypoxia/Acidosis. In Obstetric Emergencies; Springer: Singapore, 2017; pp. 7–25. [Google Scholar] [CrossRef]
- Kelly, R.; Ramaiah, S.M.; Sheridan, H.; Cruickshank, H.; Rudnicka, M.; Kissack, C.; Becher, J.-C.; Stenson, B.J. Dose-dependent relationship between acidosis at birth and likelihood of death or cerebral palsy. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 103, F567–F572. [Google Scholar] [CrossRef]
- Gee, S.E.; Frey, H.A. Contractions: Traditional concepts and their role in modern obstetrics. Semin. Perinatol. 2020, 44, 151218.
- Pla, L.; Berdún, S.; Mir, M.; Rivas, L.; Miserere, S.; Dulay, S.; Samitier, J.; Eixarch, E.; Illa, M.; Gratacós, E. Non-invasive monitoring of pH and oxygen using miniaturized electrochemical sensors in an animal model of acute hypoxia. J. Transl. Med. 2021, 19, 53. [Google Scholar] [CrossRef]
- Abdulhay, E.W.; Oweis, R.J.; Alhaddad, A.M.; Sublaban, F.N.; Radwan, M.A. Non-Invasive Fetal Heart Rate Monitoring Techniques: Review Article. Biomed. Sci. Eng. 2014, 2, 53–67.
- Jakes, A.D.; Ali, M.; Lloyd, J. Fetal Scalp Blood Sampling. Found. Years J. 2017, 11, 77–81. Available online: https://www.researchgate.net/publication/318654298_Fetal_Scalp_Blood_Sampling (accessed on 10 March 2021).
- Carbonne, B.; Pons, K.; Maisonneuve, E. Foetal scalp blood sampling during labour for pH and lactate measurements. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 30, 62–67.
- Fetal Capillary Blood pH (Fetal Blood Sampling). Available online: https://acutecaretesting.org/en/articles/fetal-capillary-blood-ph-fetal-blood-sampling (accessed on 10 March 2021).
- Queensland Clinical Guidelines. Maternity and Neonatal Clinical Guideline, Intrapartum Fetal Surveillance (IFS); Queensland Clinical Guidelines; Queensland Health: Herston, QLD, Australia, 2019; pp. 1–29.
- Sarkawi, S.; Zakaria, Z.; Balkhis, I.; Jalil, J.A.; Rahim, M.A.A.; Rahiman, M.H.F.; Mustafa, N.; Rahim, R.A.; Shaffie, Z. 3D model simulation on magnetic induction spectroscopy for fetal acidosis detection using COMSOL multiphysics. AIP Conf. Proc. 2016, 1774, 050003.
- Deans, A.; Coxon, S.; Kirkpatrick, A.; Coxon, S.; Jones, Z. Intrapartum Fetal Heart Monitoring Guideline. Frimley Health, NHS Foundation Trust. 2020. Available online: https://www.frimleyhealthandcare.org.uk/media/2178/fetal-monitoring-including-fetal-blood-sampling.pdf (accessed on 19 August 2021).
- Henderson, Z.; Ecker, J.L. Fetal Scalp Blood Sampling—Limited Role in Contemporary Obstetric Practice: Part I. Lab. Med. 2003, 34, 548–553.
- Fetal Scalp Blood Sampling; Obstetrics and Gynaecology Clinical Practice Guideline WNHS, North Metropolitan Health Service Australia. 2021; pp. 1–5. Available online: https://www.kemh.health.wa.gov.au/~/media/HSPs/NMHS/Hospitals/WNHS/Documents/Clinical-guidelines/Obs-Gyn-Guidelines/Fetal-scalp-blood-sampling (accessed on 19 August 2021).
- Ancillary Tests. Available online: https://www.brainkart.com/article/Ancillary-Tests_25669/ (accessed on 10 August 2021).
- Demaegd, H.M.I.; Bauters, E.G.R.; Page, G.H. Foetal scalp blood sampling and ST-analysis of the foetal ECG for intrapartum foetal monitoring: A restricted systematic review. Facts Views Vis. ObGyn 2020, 11, 337–346.
- Blix, E.; Maude, R.; Hals, E.; Kisa, S.; Karlsen, E.; Nohr, E.A.; de Jonge, A.; Lindgren, H.; Downe, S.; Reinar, L.M.; et al. Intermittent auscultation fetal monitoring during labour: A systematic scoping review to identify methods, effects, and accuracy. PLoS ONE 2019, 14, e0219573.
- History of Fetal Monitoring–Electronic Fetal Monitoring. Available online: http://www.ob-efm.com/efm-basics/history/ (accessed on 11 March 2021).
- East, C.E.; Begg, L.; Colditz, P.B.; Lau, R. Fetal pulse oximetry for fetal assessment in labour (Review). Cochrane Database Syst. Rev. 2014, 14, CD004075.
- Fong, D.D.; Yamashiro, K.J.; Vali, K.; Galganski, L.A.; Thies, J.; Moeinzadeh, R.; Pivetti, C.; Knoesen, A.; Srinivasan, V.J.; Hedriana, H.L.; et al. Design and In Vivo Evaluation of a Non-Invasive Transabdominal Fetal Pulse Oximeter. IEEE Trans. Biomed. Eng. 2021, 68, 256–266.
- Manuel, J. Complexity Sciences Applied to Cardiotocography; Faculty of Medicine, University of Porto: Porto, Portugal, 2020; pp. 1–89. Available online: https://repositorio-aberto.up.pt/handle/10216/132246 (accessed on 10 March 2021).
- Ekengård, F.; Cardell, M.; Herbst, A. Impaired validity of the new FIGO and Swedish CTG classification templates to identify fetal acidosis in the first stage of labor. J. Matern. Neonatal Med. 2021, 1–8.
- Popescu, M.R.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Peltecu, G.; Zagrean, A.-M. Getting an Early Start in Understanding Perinatal Asphyxia Impact on the Cardiovascular System. Front. Pediatr. 2020, 8, 68.
- Wu, W.; Wang, H.; Zhao, P.; Talcott, M.; Lai, S.; McKinstry, R.C.; Woodard, P.K.; Macones, G.A.; Schwartz, A.L.; Cahill, A.G.; et al. Noninvasive high-resolution electromyometrial imaging of uterine contractions in a translational sheep model. Sci. Transl. Med. 2019, 11, eaau1428.
