The aquaporins (AQPs) are a family of small integral membrane proteins that facilitate the bidirectional transport of water across biological membranes in response to osmotic pressure gradients as well as enabling the transmembrane diffusion of small neutral solutes (such as urea, glycerol, and hydrogen peroxide) and ions. AQPs are expressed throughout the human body.
- aquaporin (AQP)
- membranes
- water
- fluid
- secretion
1. Introduction
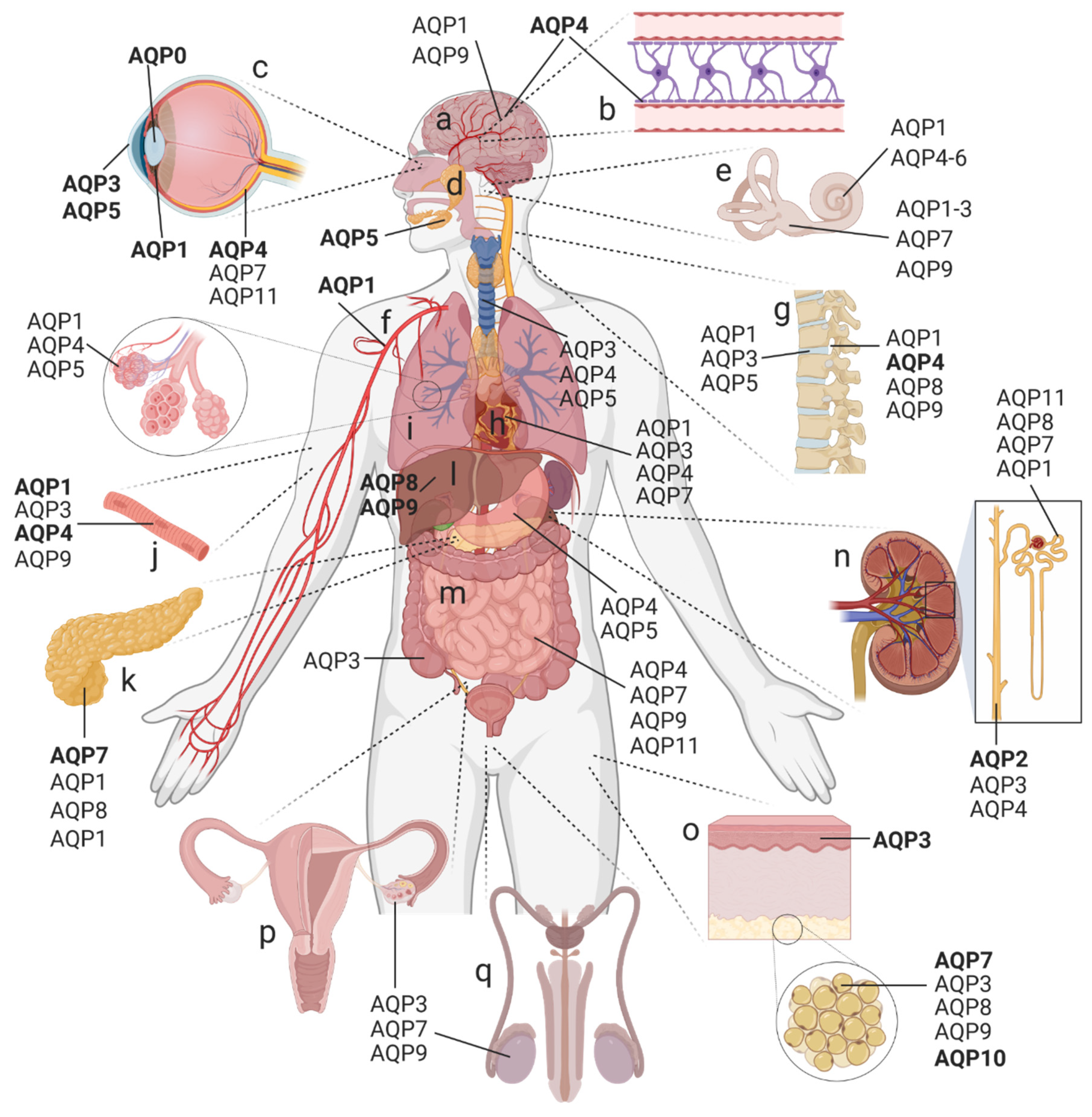
2. Distribution and Classification of AQPs in the Human Body
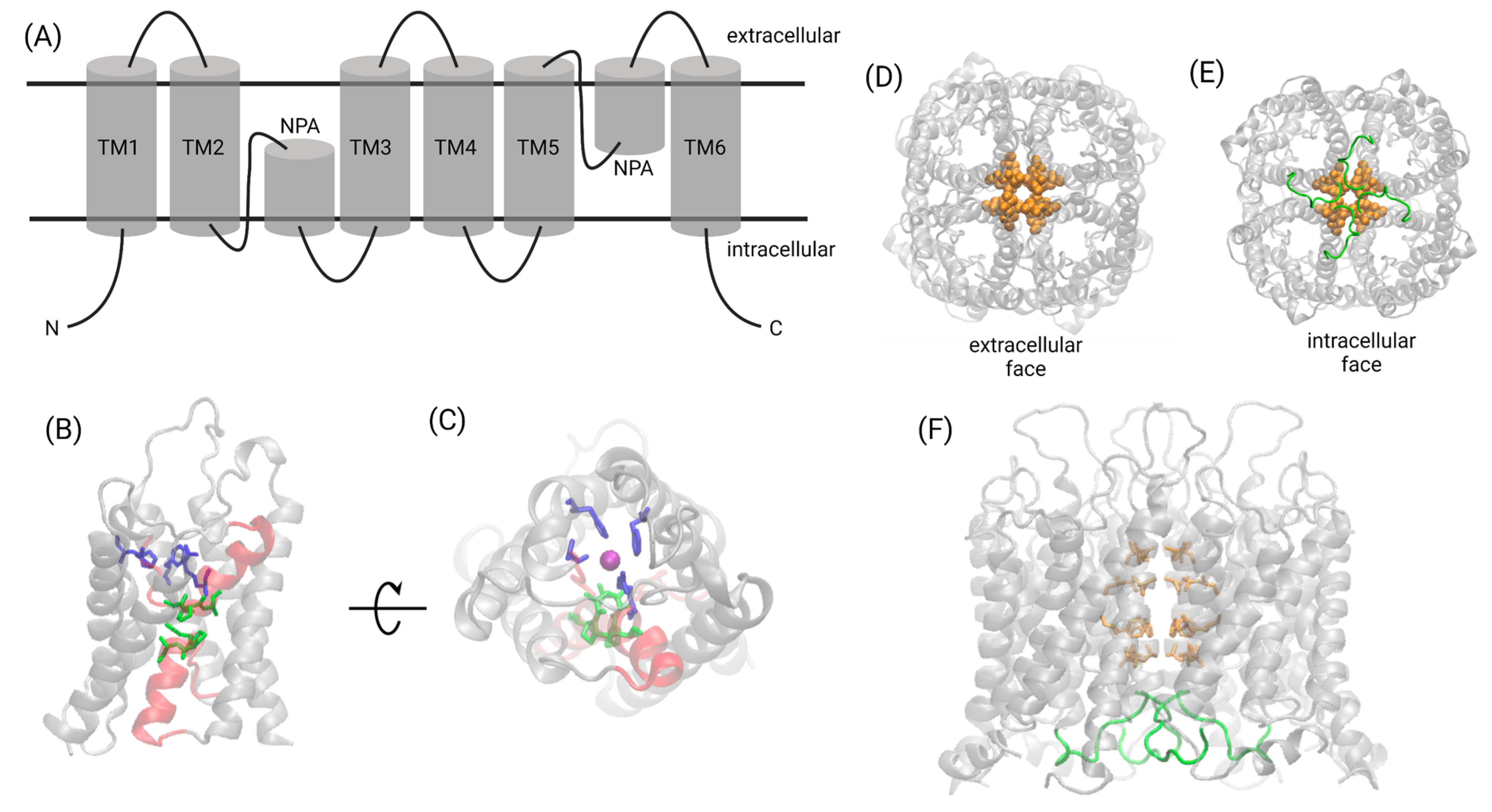
3. Structural Biology of the AQP Family
4. AQP Permeabilities: An Expanding Repertoire
|
Aquaporin |
Chromosome |
Water Permeability (Pf) [×10−14 cm3 s−1] |
Permeability to Molecules Other Than Water |
Main Expression Sites |
|---|---|---|---|---|
|
Orthodox (classical) AQPs |
||||
|
AQP0 |
12q13 |
0.25 |
Eye lens |
|
|
AQP1 |
7p14 |
6.0 |
Monovalent cations [24][36][42], nitric oxide [70], H2O2 [49][55], and glycerol * [71] |
Central nervous system (CNS), inner ear, eye, kidney, endothelium, lung, skeletal muscle, cartilage, and erythrocytes |
|
AQP2 |
12q13 |
3.3 |
None known |
Kidney, inner ear, and reproductive tract |
|
AQP4 |
18q22 |
24 |
Nitric oxide [72] |
CNS, inner ear, retina, kidney, gastrointestinal tract (GIT), lung, and skeletal muscle |
|
AQP5 |
12q13 |
5.0 |
H2O2 [51] |
Secretory glands, inner ear, eye, kidney, GIT, and lung |
|
AQP6 |
12q13 |
Low; no quantitative data |
Ammonia [73], glycerol, urea [74], nitrate [75], and anions (NO3−, Cl−) [76] |
Inner ear, kidney |
|
AQP8 |
16p12 |
No quantitative data |
Urea, ammonia, and H2O2 [77] |
Liver, kidney, adipose tissue, pancreas, GIT, and reproductive tract |
|
Aquaglyceroporins |
||||
|
AQP3 |
9p13 |
2.1 |
Skin, inner ear, eye, adipose tissue, kidney, GIT, heart, lung, reproductive tract, and cartilage |
|
|
AQP7 |
9p13 |
No quantitative data |
Adipose tissue, pancreas, liver, kidney, inner ear, GIT, heart, reproductive tract |
|
|
AQP9 |
15q22 |
No quantitative data |
Arsenite [80], carbamides, polyols, purines, pyrimidines [83], ketone bodies [84], lactate [85], ammonia [86], glycerol, urea [83][87][88], and H2O2 [54] |
Liver, adipose tissue, CNS (unclear for humans), inner ear, and reproductive tract |
|
AQP10 |
1q21 |
No quantitative data |
Glycerol [89] |
Adipose tissue and reproductive tract |
|
Unorthodox AQPs/S-aquaporins |
||||
|
AQP11 |
11q13 |
~2 |
Retina, kidney, GIT, and reproductive tract |
|
|
AQP12 |
2q37 |
No quantitative data |
Unknown |
Pancreas |

This entry is adapted from the peer-reviewed paper 10.3390/ijms23031388
References
- Salman, M.M.; Kitchen, P.; Yool, A.J.; Bill, R.M. Recent breakthroughs and future directions in drugging aquaporins. Trends Pharm. Sci. 2022, 43, 30–42.
- Benga, G.; Popescu, O.; Borza, V.; Pop, V.I.; Muresan, A.; Mocsy, I.; Brain, A.; Wrigglesworth, J.M. Water permeability in human erythrocytes: Identification of membrane proteins involved in water transport. Eur. J. Cell Biol. 1986, 41, 252–262.
- Benga, G.; Popescu, O.; Pop, V.I.; Holmes, R.P. p-(Chloromercuri)benzenesulfonate binding by membrane proteins and the inhibition of water transport in human erythrocytes. Biochemistry 1986, 25, 1535–1538.
- Preston, G.M.; Agre, P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: Member of an ancient channel family. Proc. Natl. Acad. Sci. USA 1991, 88, 11110–11114.
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387.
- Gorin, M.B.; Yancey, S.B.; Cline, J.; Revel, J.P.; Horwitz, J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: Characterization and structure based on cDNA cloning. Cell 1984, 39, 49–59.
- Zampighi, G.A.; Hall, J.E.; Kreman, M. Purified lens junctional protein forms channels in planar lipid films. Proc. Natl. Acad. Sci. USA 1985, 82, 8468–8472.
- Chandy, G.; Zampighi, G.A.; Kreman, M.; Hall, J.E. Comparison of the water transporting properties of MIP and AQP1. J. Membr. Biol. 1997, 159, 29–39.
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604.
- Madeira, A.; Moura, T.F.; Soveral, G. Aquaglyceroporins: Implications in adipose biology and obesity. Cell. Mol. Life Sci. 2015, 72, 759–771.
- Yool, A.J.; Campbell, E.M. Structure, function and translational relevance of aquaporin dual water and ion channels. Mol. Aspects Med. 2012, 33, 553–561.
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta 2014, 1840, 1492–1506.
- Tyerman, S.D.; McGaughey, S.A.; Qiu, J.; Yool, A.J.; Byrt, C.S. Adaptable and Multifunctional Ion-Conducting Aquaporins. Annu. Rev. Plant Biol. 2021, 72, 703–736.
- Kourghi, M.; Nourmohammadi, S.; Pei, J.V.; Qiu, J.; McGaughey, S.; Tyerman, S.D.; Byrt, C.S.; Yool, A.J. Divalent Cations Regulate the Ion Conductance Properties of Diverse Classes of Aquaporins. Int. J. Mol. Sci. 2017, 18, 2323.
- Weaver, C.D.; Shomer, N.H.; Louis, C.F.; Roberts, D.M. Nodulin 26, a nodule-specific symbiosome membrane protein from soybean, is an ion channel. J. Biol. Chem. 1994, 269, 17858–17862.
- Hwang, J.H.; Ellingson, S.R.; Roberts, D.M. Ammonia permeability of the soybean nodulin 26 channel. FEBS Lett. 2010, 584, 4339–4343.
- Gonen, T.; Walz, T. The structure of aquaporins. Q. Rev. Biophys. 2006, 39, 361–396.
- Krenc, D.; Song, J.; Almasalmeh, A.; Wu, B.; Beitz, E. The arginine-facing amino acid residue of the rat aquaporin 1 constriction determines solute selectivity according to its size and lipophilicity. Mol. Membr. Biol. 2014, 31, 228–238.
- Kushmerick, C.; Rice, S.J.; Baldo, G.J.; Haspel, H.C.; Mathias, R.T. Ion, water and neutral solute transport in Xenopus oocytes expressing frog lens MIP. Exp. Eye Res. 1995, 61, 351–362.
- Ehring, G.R.; Zampighi, G.; Horwitz, J.; Bok, D.; Hall, J.E. Properties of channels reconstituted from the major intrinsic protein of lens fiber membranes. J. Gen. Physiol. 1990, 96, 631–664.
- Yool, A.J.; Stamer, W.D.; Regan, J.W. Forskolin stimulation of water and cation permeability in aquaporin 1 water channels. Science 1996, 273, 1216–1218.
- Saparov, S.M.; Kozono, D.; Rothe, U.; Agre, P.; Pohl, P. Water and ion permeation of aquaporin-1 in planar lipid bilayers. Major differences in structural determinants and stoichiometry. J. Biol. Chem. 2001, 276, 31515–31520.
- Anthony, T.L.; Brooks, H.L.; Boassa, D.; Leonov, S.; Yanochko, G.M.; Regan, J.W.; Yool, A.J. Cloned human aquaporin-1 is a cyclic GMP-gated ion channel. Mol. Pharmacol. 2000, 57, 576–588.
- Campbell, E.M.; Birdsell, D.N.; Yool, A.J. The activity of human aquaporin 1 as a cGMP-gated cation channel is regulated by tyrosine phosphorylation in the carboxyl-terminal domain. Mol. Pharmacol. 2012, 81, 97–105.
- Yanochko, G.M.; Yool, A.J. Regulated cationic channel function in Xenopus oocytes expressing Drosophila big brain. J. Neurosci. 2002, 22, 2530–2540.
- Yasui, M.; Hazama, A.; Kwon, T.H.; Nielsen, S.; Guggino, W.B.; Agre, P. Rapid gating and anion permeability of an intracellular aquaporin. Nature 1999, 402, 184–187.
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta 2014, 1840, 1507–1512.
- Gorelick, D.A.; Praetorius, J.; Tsunenari, T.; Nielsen, S.; Agre, P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006, 7, 14.
- Yakata, K.; Hiroaki, Y.; Ishibashi, K.; Sohara, E.; Sasaki, S.; Mitsuoka, K.; Fujiyoshi, Y. Aquaporin-11 containing a divergent NPA motif has normal water channel activity. Biochim. Biophys. Acta 2007, 1768, 688–693.
- Calvanese, L.; Pellegrini-Calace, M.; Oliva, R. In silico study of human aquaporin AQP11 and AQP12 channels. Protein Sci. 2013, 22, 455–466.
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799.
- Frick, A.; Eriksson, U.K.; de Mattia, F.; Oberg, F.; Hedfalk, K.; Neutze, R.; de Grip, W.J.; Deen, P.M.; Tornroth-Horsefield, S. X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc. Natl. Acad. Sci. USA 2014, 111, 6305–6310.
- Jan, L.Y.; Jan, Y.N. Structural Elements Involved in Specific K+ Channel Functions. Annu. Rev. Physiol. 1992, 54, 537–555.
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605.
- Walz, T.; Hirai, T.; Murata, K.; Heymann, J.B.; Mitsuoka, K.; Fujiyoshi, Y.; Smith, B.L.; Agre, P.; Engel, A. The three-dimensional structure of aquaporin-1. Nature 1997, 387, 624–627.
- Yool, A.J.; Weinstein, A.M. New roles for old holes: Ion channel function in aquaporin-1. News Physiol. Sci. 2002, 17, 68–72.
- Sui, H.; Han, B.G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature 2001, 414, 872–878.
- Fu, D.; Libson, A.; Miercke, L.J.; Weitzman, C.; Nollert, P.; Krucinski, J.; Stroud, R.M. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 2000, 290, 481–486.
- Ho, J.D.; Yeh, R.; Sandstrom, A.; Chorny, I.; Harries, W.E.; Robbins, R.A.; Miercke, L.J.; Stroud, R.M. Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance. Proc. Natl. Acad. Sci. USA 2009, 106, 7437–7442.
- Mathai, J.C.; Agre, P. Hourglass pore-forming domains restrict aquaporin-1 tetramer assembly. Biochemistry 1999, 38, 923–928.
- Kitchen, P.; Conner, M.T.; Bill, R.M.; Conner, A.C. Structural Determinants of Oligomerization of the Aquaporin-4 Channel. J. Biol. Chem. 2016, 291, 6858–6871.
- Yu, J.; Yool, A.J.; Schulten, K.; Tajkhorshid, E. Mechanism of gating and ion conductivity of a possible tetrameric pore in aquaporin-1. Structure 2006, 14, 1411–1423.
- Kourghi, M.; De Ieso, M.L.; Nourmohammadi, S.; Pei, J.V.; Yool, A.J. Identification of Loop D Domain Amino Acids in the Human Aquaporin-1 Channel Involved in Activation of the Ionic Conductance and Inhibition by AqB011. Front. Chem. 2018, 6, 142.
- Endeward, V.; Musa-Aziz, R.; Cooper, G.J.; Chen, L.M.; Pelletier, M.F.; Virkki, L.V.; Supuran, C.T.; King, L.S.; Boron, W.F.; Gros, G. Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J. 2006, 20, 1974–1981.
- Boassa, D.; Yool, A.J. A fascinating tail: cGMP activation of aquaporin-1 ion channels. Trends Pharmacol. Sci. 2002, 23, 558–562.
- Boassa, D.; Yool, A.J. Single amino acids in the carboxyl terminal domain of aquaporin-1 contribute to cGMP-dependent ion channel activation. BMC Physiol. 2003, 3, 12.
- Hazama, A.; Kozono, D.; Guggino, W.B.; Agre, P.; Yasui, M. Ion permeation of AQP6 water channel protein. Single channel recordings after Hg2+ activation. J. Biol. Chem. 2002, 277, 29224–29230.
- Liu, K.; Kozono, D.; Kato, Y.; Agre, P.; Hazama, A.; Yasui, M. Conversion of aquaporin 6 from an anion channel to a water-selective channel by a single amino acid substitution. Proc. Natl. Acad. Sci. USA 2005, 102, 2192–2197.
- Montiel, V.; Bella, R.; Michel, L.Y.M.; Esfahani, H.; De Mulder, D.; Robinson, E.L.; Deglasse, J.-P.; Tiburcy, M.; Chow, P.H.; Jonas, J.-C.; et al. Inhibition of aquaporin-1 prevents myocardial remodeling by blocking the transmembrane transport of hydrogen peroxide. Sci. Transl. Med. 2020, 12, eaay2176.
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686.
- Rodrigues, C.; Pimpão, C.; Mósca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human Aquaporin-5 Facilitates Hydrogen Peroxide Permeation Affecting Adaption to Oxidative Stress and Cancer Cell Migration. Cancers 2019, 11, 932.
- Marchissio, M.J.; Francés, D.E.; Carnovale, C.E.; Marinelli, R.A. Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells causes ROS-induced mitochondrial depolarization and loss of viability. Toxicol Appl. Pharm. 2012, 264, 246–254.
- Bertolotti, M.; Bestetti, S.; García-Manteiga, J.M.; Medraño-Fernandez, I.; Dal Mas, A.; Malosio, M.L.; Sitia, R. Tyrosine kinase signal modulation: A matter of H2O2 membrane permeability? Antioxid Redox Signal 2013, 19, 1447–1451.
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197.
- Almasalmeh, A.; Krenc, D.; Wu, B.; Beitz, E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 2014, 281, 647–656.
- Kitchen, P.; Day, R.E.; Salman, M.M.; Conner, M.T.; Bill, R.M.; Conner, A.C. Beyond water homeostasis: Diverse functional roles of mammalian aquaporins. Biochim. Biophys. Acta 2015, 1850, 2410–2421.
- Lv, H.; Li, Y.; Xue, C.; Dong, N.; Bi, C.; Shan, A. Aquaporin: Targets for dietary nutrients to regulate intestinal health. J. Anim. Physiol. Anim. Nutr. 2021, 106, 167–180.
- Olesen, E.T.B.; Fenton, R.A. Aquaporin 2 regulation: Implications for water balance and polycystic kidney diseases. Nat. Rev. Nephrol. 2021, 17, 765–781.
- Tardelli, M.; Stulnig, T.M. Aquaporin regulation in metabolic organs. Vitam. Horm. 2020, 112, 71–93.
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the epidermis: More than skin deep. Am. J. Physiol. Cell Physiol. 2020, 318, C1144–C1153.
- Mogensen, F.L.; Delle, C.; Nedergaard, M. The Glymphatic System (En)during Inflammation. Int. J. Mol. Sci. 2021, 22, 7491.
- Noda, Y.; Sasaki, S. Updates and Perspectives on Aquaporin-2 and Water Balance Disorders. Int. J. Mol. Sci. 2021, 22, 12950.
- Valenti, G.; Tamma, G. The vasopressin-aquaporin-2 pathway syndromes. Handb. Clin. Neurol. 2021, 181, 249–259.
- Salman, M.M.; Kitchen, P.; Iliff, J.J.; Bill, R.M. Aquaporin 4 and glymphatic flow have central roles in brain fluid homeostasis. Nat. Rev. Neurosci. 2021, 22, 650–651.
- Salman, M.M.; Kitchen, P.; Halsey, A.; Wang, M.X.; Tornroth-Horsefield, S.; Conner, A.C.; Badaut, J.; Iliff, J.J.; Bill, R.M. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 2021.
- Markou, A.; Unger, L.; Abir-Awan, M.; Saadallah, A.; Halsey, A.; Balklava, Z.; Conner, M.; Törnroth-Horsefield, S.; Greenhill, S.D.; Conner, A.; et al. Molecular mechanisms governing aquaporin relocalisation. Biochim. Biophys. Acta Biomembr. 2021, 1864, 183853.
- De Ieso, M.L.; Yool, A.J. Mechanisms of Aquaporin-Facilitated Cancer Invasion and Metastasis. Front. Chem. 2018, 6, 135.
- Yool, A.J.; Ramesh, S. Molecular Targets for Combined Therapeutic Strategies to Limit Glioblastoma Cell Migration and Invasion. Front. Pharm. 2020, 11, 358.
- Castle, N. Aquaporins as targets for drug discovery. Drug Discov. Today 2005, 10, 485–493.
- Herrera, M.; Hong, N.J.; Garvin, J.L. Aquaporin-1 transports NO across cell membranes. Hypertension 2006, 48, 157–164.
- Abrami, L.; Tacnet, F.; Ripoche, P. Evidence for a glycerol pathway through aquaporin 1 (CHIP28) channels. Pflügers Archiv 1995, 430, 447–458.
- Wang, Y.; Tajkhorshid, E. Nitric oxide conduction by the brain aquaporin AQP4. Proteins 2010, 78, 661–670.
- Soria, L.R.; Fanelli, E.; Altamura, N.; Svelto, M.; Marinelli, R.A.; Calamita, G. Aquaporin-8-facilitated mitochondrial ammonia transport. Biochem. Biophys. Res. Commun. 2010, 393, 217–221.
- Holm, L.M.; Klaerke, D.A.; Zeuthen, T. Aquaporin 6 is permeable to glycerol and urea. Pflügers Archiv 2004, 448, 181–186.
- Ikeda, M.; Beitz, E.; Kozono, D.; Guggino, W.B.; Agre, P.; Yasui, M. Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J. Biol. Chem. 2002, 277, 39873–39879.
- Rambow, J.; Wu, B.; Rönfeldt, D.; Beitz, E. Aquaporins with anion/monocarboxylate permeability: Mechanisms, relevance for pathogenic “host interactions. Front. Pharmacol. 2014, 5, 199.
- Chauvigné, F.; Yilmaz, O.; Ferré, A.; Fjelldal, P.G.; Finn, R.N.; Cerdà, J. The vertebrate Aqp14 water channel is a neuropeptide-regulated polytransporter. Commun. Biol. 2019, 2, 462.
- Ishibashi, K.; Sasaki, S.; Fushimi, K.; Uchida, S.; Kuwahara, M.; Saito, H.; Furukawa, T.; Nakajima, K.; Yamaguchi, Y.; Gojobori, T.; et al. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc. Natl. Acad. Sci. USA 1994, 91, 6269–6273.
- Soveral, G.; Nielsen, S.; Casini, A. Aquaporins in Health and Disease: New Molecular Targets for Drug Discovery; Taylor Francis Group (CRC Press): Boca Raton, FL, USA, 2016.
- Liu, Z.; Shen, J.; Carbrey, J.M.; Mukhopadhyay, R.; Agre, P.; Rosen, B.P. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. USA 2002, 99, 6053–6058.
- Ishibashi, K.; Kuwahara, M.; Gu, Y.; Kageyama, Y.; Tohsaka, A.; Suzuki, F.; Marumo, F.; Sasaki, S. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J. Biol. Chem. 1997, 272, 20782–20786.
- Geyer, R.R.; Musa-Aziz, R.; Qin, X.; Boron, W.F. Relative CO(2)/NH(3) selectivities of mammalian aquaporins 0-9. Am. J. Physiol. Cell Physiol. 2013, 304, C985–C994.
- Tsukaguchi, H.; Shayakul, C.; Berger, U.V.; Mackenzie, B.; Devidas, S.; Guggino, W.B.; van Hoek, A.N.; Hediger, M.A. Molecular characterization of a broad selectivity neutral solute channel. J. Biol. Chem. 1998, 273, 24737–24743.
- Elkjaer, M.; Vajda, Z.; Nejsum, L.N.; Kwon, T.; Jensen, U.B.; Amiry-Moghaddam, M.; Frøkiaer, J.; Nielsen, S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem. Biophys. Res. Commun. 2000, 276, 1118–1128.
- Akashi, A.; Miki, A.; Kanamori, A.; Nakamura, M. Aquaporin 9 expression is required for l-lactate to maintain retinal neuronal survival. Neurosci. Lett. 2015, 589, 185–190.
- Stahl, K.; Rahmani, S.; Prydz, A.; Skauli, N.; MacAulay, N.; Mylonakou, M.N.; Torp, R.; Skare, Ø.; Berg, T.; Leergaard, T.B.; et al. Targeted deletion of the aquaglyceroporin AQP9 is protective in a mouse model of Parkinson’s disease. PLoS ONE 2018, 13, e0194896.
- Ishibashi, K.; Kuwahara, M.; Gu, Y.; Tanaka, Y.; Marumo, F.; Sasaki, S. Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol. Biochem. Biophys. Res. Commun. 1998, 244, 268–274.
- Tsukaguchi, H.; Weremowicz, S.; Morton, C.C.; Hediger, M.A. Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am. J. Physiol. 1999, 277, F685–F696.
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 Represents an Alternative Pathway for Glycerol Efflux from Human Adipocytes. PLoS ONE 2013, 8, e54474.
- Madeira, A.; Fernández-Veledo, S.; Camps, M.; Zorzano, A.; Moura, T.F.; Ceperuelo-Mallafré, V.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017.
- Finn, R.N.; Cerdà, J. Evolution and functional diversity of aquaporins. Biol. Bull. 2015, 229, 6–23.
