Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
In the new era of modern flexible and bendable technology, graphene-based materials have attracted tremendous attention. The excellent electrical, mechanical, and optical properties of graphene as well as the ease of functionalization of its derivates enable graphene to become an attractive candidate in the construction of flexible devices.
- graphene
- flexible devices
- composite
- graphene oxide
1. Introduction
It is well known that materials play an important role in the development of science and technology, because the realization of a new technology often requires the support of novel materials. Therefore, exploring materials with excellent properties has always been an important subject of scientific research. A remarkable material, graphene, has attracted widespread attention since it was first exfoliated from graphite by Andre Geim and Konstantin Novoselov in 2004. As a result of its prominent performances, graphene can be used in various fields, such as energy storage, biosensing, optoelectronics, flexible electronics, electrochemical sensing, robotics, textile industry, and so on [1][2][3][4]. The discovery of graphene marked the beginning of a new era in material science research [5].
Graphene with a thickness of a single carbon atom is arranged in a honeycomb lattice. It is very solid and can be fashioned into 0D, 1D, 3D forms (Figure 1) [6]. In addition, it is extraordinary transparent and possesses high crystallite as well as outstanding electronic properties. Although graphene has many excellent properties, there is no bandgap in graphene, and it has poor water solubility, which greatly limits its application in some areas [7]. An effective way to overcome these limitations and expand the range of application of graphene is to prepare graphene derivatives. For example, treating graphite with strong oxidants will add epoxy groups, hydroxyl groups, and carboxyl group on the basal plan of graphite layers, thus producing graphene oxide (GO). These polar oxygen-containing functional groups make GO highly hydrophilic. This allows GO to have excellent dispersibility in many solvents, especially in water. In addition, the oxygen-containing functional groups can provide reactive sites for chemical modification or functionalization of GO, which in turn can be used to develop GO-based materials. Although the oxygen-containing groups can obviate some disadvantages of graphene, they also cause some problems. For example, they make GO electrically insulating. Nevertheless, the chemical reduction of GO can restore its conductivity to some extent. The obtained reduced GO (rGO) still carries some functional groups, which results in a good dispersion of rGO in many solvents. Most importantly, it is relatively easy to control the electrical performance and solubility of rGO by controlling the number of the remaining functional groups. The properties of this chemically reduced graphene approximately resemble those of pristine graphene [6][8]. The transformation of graphite to graphite oxide, GO and graphene is shown in Figure 2.
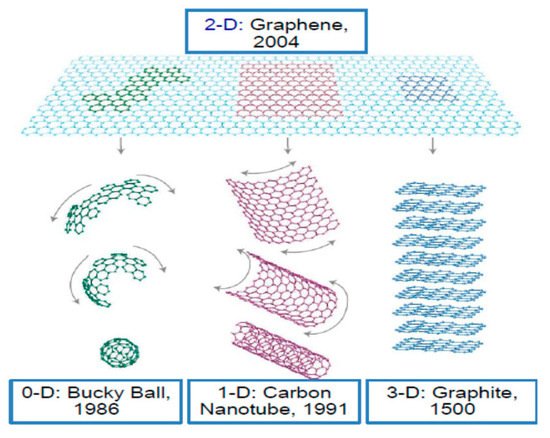
Figure 1. Various structures of graphene (0D bucky ball, 1D carbon nanotube, 3D graphite). (Reproduced with permission from ref. [9]. Copyright 2016 Springer Publications).

Figure 2. Schematic of the transformation of graphite oxide to GO and graphene. (Reproduced with permission from ref. [6]. Copyright 2016 SAGE Publications).
Graphene and its derivatives have their own unique advantages and can be used in many domains applying different techniques, such as thermal chemical vapor deposition (CVD), self-assembly technique, spin coating, vacuum filtration, thermal decomposition, solution dispersion technique, and chemical decomposition polymer processing technique [8][10][11][12][13][14][15].
2. Synthesis of Graphene and Its Derivatives
2.1. GO
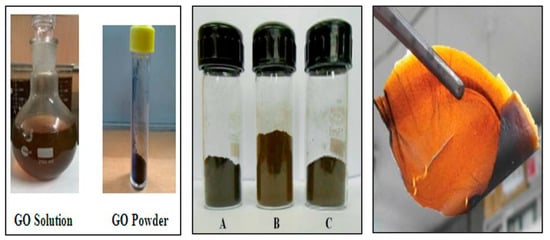
There are many reports about the synthesis of GO, and the structures of the obtained products are slightly different (Figure 3). One of the most classical methods was proposed by Williams Hummers JR and Richard Offeman in 1958. The general process was as follows. Graphite was first mixed with concentrated sulfuric acid and oxidizers such as sodium nitrate, then potassium permanganate was added under a precise temperature control, followed by the addition of reducing and reaction stopping agents such as hydrogen peroxide at the end of the process [16]. This method supplies a high yield of colloidal suspension and powdery product [17]. Later, numerous research groups made further improvements of the preparation method focusing on three main parameters, i.e., precursors ratio, time, and temperature [18]. For example, Marcano et al. synthesized GO by using the Tours method and obtained high-quality GO by adding phosphoric acid as a key precursor and removing sodium nitrate with the product. This method is better than previous methods due to its simplicity and outstanding product quality [19]. In addition to these three parameters, the size of graphite particle also has a great effect on the quality of the final products [20]. According to the demands of different applications, various physical forms of GO such as suspension, powder, and flexible sheet can be prepared. The corresponding photos are shown in Figure 4.
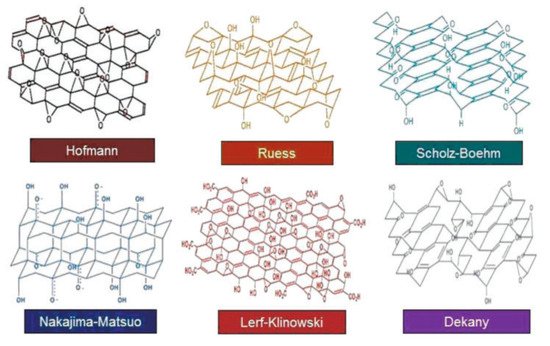
Figure 3. Different structures of GO. (Reproduced with permission from ref. [6]. Copyright 2016 SAGE Publications).
2.2. Graphene
Several attempts have been adopted to study the synthesis methods of carbon-based materials. The very first attempt dates back to 1962. Boehm et al. prepared soot composed of thin-layer graphite-intercalated compounds by the reduction and combustion of graphite oxide. In 1944, these products were named as graphene platelets and had a single carbon layer [22]. In 2004, Novoselov et al. obtained graphene by the scotch tape method and won the Noble Prize in 2010 [23]. Till now, the preparation methods of graphene include top-down and bottom-up techniques. Top-down methods include scotch tape exfoliation, liquid-phase exfoliation, and chemical synthesis. Bottom-up methods mainly comprise CVD and molecular beam epitaxy [24][25][26]. In this section, typical methods of graphene synthesis will be introduced.
2.3. rGO
GO prepared by Hummer’s method consists of a-few-layer carbon platelets decorated with oxygen containing functional groups. The removal of some oxygen-based groups by reducing agents or thermal treatment can yield rGO (Figure 5). The main process is as follows. GO is exfoliated via ultrasonication and then reduced by hydrazine hydrate, a strong reducing agent, for 2 h. Since hydrazine is toxic, alternative reagents such as NaBH4, ascorbic acid, and HI can be used. Among these, ascorbic acid is essential for the scalable production of rGO. The chemical procedure to obtain rGO using ascorbic acid as a reducing agent is shown in Figure 6. This reaction does not produce toxic gases [27]. rGO has been proven to be a good candidate for various applications such as field effect transistors (FET), solar cells, energy applications, and production of composite paper-like materials [28] due to its abundant atomic defects.
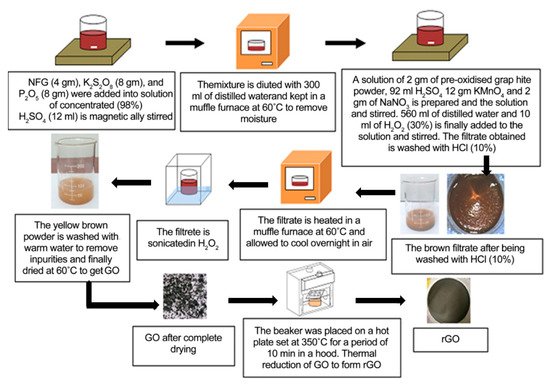
Figure 5. Steps of the synthesis of GO and rGO. (Reproduced with permission from ref. [27]. Copyright 2017 Scientific Research Publications).

Figure 6. Consecutive steps in the chemical synthesis of rGO using ascorbic acid as a reducing agent. (a) Oxidation and exfoliation of graphite using Hummer’s method. (b) Reduction and conversion of Mn (VII) ions to soluble Mn (II) ions by the addition of ascorbic acid. (c) Color transition of the exfoliated graphite oxide from greenish yellow to black in the early stage of reduction. (d) Loss of hydrophilicity of GO when stirring is paused. (e) Precipitation of rGO after completion of the reduction stage and cooling down to room temperature. (f) Filtration of rGO using cellulose filter paper. (g) rGO powder after freeze-drying. (Reproduced with permission from ref. [29]. Copyright 2015 Springer Nature Publications).
3. Flexible Composites and Applications of GO/rGO
The designed flexible composites should have not only excellent flexibility but also a certain strength which would enable the composites to withstand external environmental factors. Recently, a layered composite of GO/PVC with large mechanical strength fabricated by the vacuum filtration method was reported [30]. These flexible composites composed of GO and rGO are useful in multiple applications including energy storage, water purification, textiles, and robotics. Polymerization was used to prepare PANI nanowires on a GO sheet composite. The obtained products showed excellent performance when used as supercapacitor electrodes [31][32]. Water purification has become another issue in the past few decades. Ongoing research has tackled this problem. For example, a GO-based TiO2 composite membrane could be used as a filtration membrane for the removal of water impurities. The composite was fabricated by vacuum filtration and allowed a moderate water purification [33]. Although chemically modified graphene or rGO itself is not very appealing in terms of its properties, these properties can be enhanced in forming composite materials with conductive polymers. For instance, an rGO/polypyrrole nanowires composite fabricated in situ showed better performances than rGO and can be used in the fabrication of portable electronic devices [34]. Yarns were used to produce an electronic textile fabric by coating rGO through electrostatic self-assembly in the presence of adhesive bovine serum albumin. The preparation of the material is shown in Figure 7 [35].
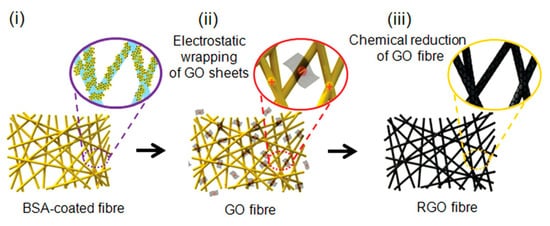
Figure 7. Illustration of the three steps used to prepare rGO/nano yarns (rGO/NYs). (Reproduced with permission from ref. [35]. Copyright 2013 Wiley Publications).
E-textile has revolutionized the whole flexible and portable device industry with the introduction of additional properties. In addition, a pressed composite of rGO–MnFe2O4 and polyvinylidene fluoride fabricated by a simple sonication method turned out to be a good absorber of harmful microwaves in the electromagnetic spectrum [36]. Within energy applications, flexible composites of V2O5/polyindole and activated carbon cloth were used as cathodic and anodic electrodes of an asymmetric supercapacitor. V2O5 nanostructures were constructed on a carbon cloth by in situ polymerization and showed good cyclic stability on testing (Figure 8) [37].
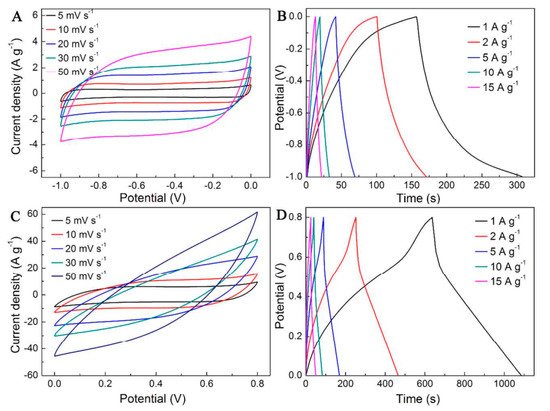
Figure 8. CVs of (A) rGO@actived carbon cloth (rGO@ACC) and (C) V2O5/polyindole@ACC. Galvanostatic charge–discharge curves of (B) rGO@ACC and (D) V2O5/polyindole@ACC. (Reproduced with permission from ref. [37]. Copyright 2016 ACS Publications).
Paper electrodes for energy application are receiving great attention. A GO solution was prepared by Hummer’s method. Then, GO-based paper electrodes which can act as flexible substrates, actuators, supercapacitor electrodes etc., were fabricated through the steps of vacuum infiltration, spin coating, and drop casting.
A composite of nickel cobalt oxide/GO was tested as a supercapacitor electrode and revealed a large capacitance of 1211 Fg-1. Its fabrication was achieved by coprecipitation using sodium dodecyl sulfate as the template and ammonia as the precipitant [38]. rGO obtained from GO by the hydrothermal route and titanium carbide obtained by selective etching of aluminum were combined by ultra-sonication and filtration, yielding the rGO/titanium carbide composite. CV, GCD, and EIS analysis proved it to be outstanding for electrochemical performance in supercapacitors [39]. Additionally, other carbon-based materials like CNT are extraordinary products due to several characteristics when hybridized with conducting polymers such as MnOx and rGO composites developed by spray coating and electrodeposition. They show a high capacitive behavior and improve the cyclic stability for supercapacitors [40]. Notably, graphene and its derivatives are analogous to other carbon-based materials, providing new perspectives to research. The fabrication techniques are also being modified. Recently, the metal-organic framework template-assisted method was utilized on a large scale and showed great potential for energy applications. In this regard, as shown in Figure 9, rGO/MoO3 was reported to be an excellent composite for energy storage in supercapacitors as an electrode [41].
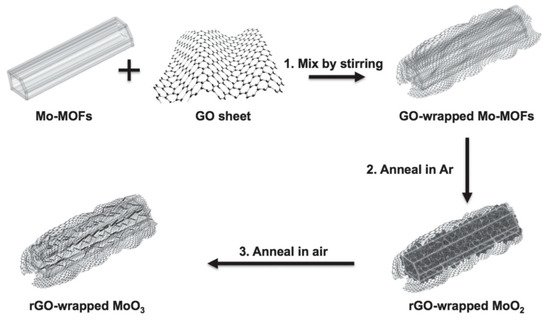
Figure 9. Preparation process of rGO/MoO3 composites. (Reproduced with permission from ref. [41]. Copyright 2015 Wiley Publications).
Organic substrates have been widely used in recent studies, but metallic substrates remain an important choice. Copper metallic foil acted as a substrate for the growth of rGO/Cu2O through the hydrothermal technique and showed moderate advantages as an electrode in supercapacitors [42]. However, the sol–gel approach is also simple and has been used for the fabrication of rGO-based composites. rGO paper was obtained by a modified Hummer’s method followed by evaporation drying. ZnO was deposited in the form of layers on rGO paper by using a stabilizer through a synthesis process. The composite ZnO/rGO/ZnO has been utilized for supercapacitor electrodes [43]. The choice of the material for positive or negative electrodes plays a vital role in energy devices. A compatible negative electrode material in a supercapacitor for Fe2O3 nanoparticle clusters/rGO paper was investigated. The composite was synthesized through the hydrothermal technique [44]. Chong et al. [45] prepared an MnO2/rGO nanocomposite by a facial one-step electrochemical method. MnO2 nanoparticles were uniformly distributed on rGO nanosheets and acted as spacers to prevent rGO nanosheets from restacking. This unique structure provided MnO2/rGO with high specific capacitance. Furthermore, the MnO2/rGO composite also showed high conductivity and excellent potential cycling stability, and has potential as electrode material for highly stable supercapacitors. In addition to MnO2, tungsten oxide (WOx) is widely studied as electrode material for supercapacitors. Recently, a W18O49 nanowires (NWs)/rGO nanocomposite, which can act as the negative electrode in asymmetric supercapacitor devices, was prepared from the precursors WCl6 and GO by the solvothermal method [46]. The asymmetric supercapacitor W18O49 NWs-rGO//rGO showed high specific capacitance and excellent cycling stability. For the fabrication of paper-based electrode, the incorporation of celluloses and pulps is desirable to attain flexibility and stability. GO-based nanocomposite of nanocrystalline cellulose acetate fabricated via stirring and a solvent casting method showed high thermal stability and good mechanical strength [47]. Likewise, cotton pulp was mixed with LiCl in addition with anhydrous DMAc by stirring. Further addition of a GO suspension to cotton pulp resulted in the formation of a cellulose-based composite useful for energy and memory storage [48]. An outstanding anodic electrode material was designed by fabricating a composite of GO and TiO2, whereas further reduction of the composite to rGO/TiO2 was obtained by stirring and drying. Anatase TiO2 exhibits higher power and energy density than other conventional metal oxides [49]. In comparison with cellulose, the residual paper pulp is more stable. As a consequence, it can be used in the fabrication of rGO-based flexible composites. First, the paper pulp was stirred in stable solvent and then it was mixed with GO. Next, the suspension was infiltrated with and reduced by hydrazine vapors at a certain temperature via the drop casting technique. The obtained composite possessed better performance compared with cellulous-based composites when applied in flexible electrodes [50]. Altogether, natural fiber-based GO/rGO paper composites have been proven to have excellent performance in multiple applications, especially in energy storage and conversion devices in the modern portable device industry.
This entry is adapted from the peer-reviewed paper 10.3390/ma15031012
References
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Shepherd, R.L.; Chen, J.; Aminorroaya–Yamini, S.; Konstantinov, K.; Minett, A.I.; Razal, J.M.; Wallace, G.G. Scalable one–step wet–spinning of graphene fibers and yarns from liquid crystalline dispersions of graphene oxide: Towards multifunctional textiles. Adv. Funct. Mater. 2013, 23, 5345–5354.
- Lundstedt, A.; Papadakis, R.; Li, H.; Han, Y.; Jorner, K.; Bergman, J.; Leifer, K.; Grennberg, H.; Ottosson, H. White–Light Photoassisted Covalent Functionalization of Graphene Using 2–Propanol. Small Methods 2017, 1, 1700214.
- Li, H.; Papadakis, R.; Jafri, S.H.M.; Thersleff, T.; Michler, J.; Ottosson, H.; Leifer, K. Superior adhesion of graphene nanoscrolls. Commun. Phys. 2018, 1, 44.
- Huang, W.; Xia, X.; Zhu, C.; Steichen, P.; Quan, W.; Mao, W.; Yang, J.; Chu, L.; Li, X. Memristive Artificial Synapses for Neuromorphic Computing. Nanomicro. Lett. 2021, 13, 85.
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596.
- Khan, Z.U.; Kausar, A.; Ullah, H.; Badshah, A.; Khan, W.U. A review of graphene oxide, graphene buckypaper, and polymer/graphene composites: Properties and fabrication techniques. J. Plast. Film Sheeting 2016, 32, 336–379.
- Geim, A.; Novoselov, K. The Rise of Graphene, Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2009; pp. 11–19.
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415.
- Sajibul, M.; Bhuyan, A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83.
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71.
- Tsou, C.-H.; An, Q.-F.; Lo, S.-C.; De Guzman, M.; Hung, W.-S.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Effect of microstructure of graphene oxide fabricated through different self-ssembly techniques on 1-butanol dehydration. J. Membr. Sci. 2015, 477, 93–100.
- Naficy, S.; Jalili, R.; Aboutalebi, S.H.; Gorkin III, R.A.; Konstantinov, K.; Innis, P.C.; Spinks, G.M.; Poulin, P.; Wallace, G.G. Graphene oxide dispersions: Tuning rheology to enable fabrication. Mater. Horiz. 2014, 1, 326–331.
- Liu, Y.; Feng, J. An attempt towards fabricating reduced graphene oxide composites with traditional polymer processing techniques by adding chemical reduction agents. Compos. Sci. Technol. 2017, 140, 16–22.
- Liu, J.; Chen, S.; Papadakis, R.; Li, H. Nanoresolution patterning of hydrogenated graphene by electron beam induced C-H dissociation. Nanotechnology 2018, 29, 415304.
- Liu, J.; Papadakis, R.; Li, H. Experimental observation of size-dependent behavior in surface energy of gold nanoparticles through atomic force microscope. Appl. Phys. Lett. 2018, 113, 083108.
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339.
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460.
- Paulchamy, B.; Arthi, G.; Lignesh, B. A simple approach to stepwise synthesis of graphene oxide nanomaterial. J. Nanomed. Nanotechnol. 2015, 6, 1.
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814.
- Chowdhury, D.R.; Singh, C.; Paul, A. Role of graphite precursor and sodium nitrate in graphite oxide synthesis. RSC Adv. 2014, 4, 15138–15145.
- Song, J.; Wang, X.; Chang, C.-T. Preparation and characterization of graphene oxide. J. Nanomater. 2014, 2014, 276143.
- Boehm, H.; Clauss, A.; Fischer, G.O.; Hofmann, U. In Surface Properties of Extremely Thin Graphite Lamellae. In Proceedings of the Fifth Conference on Carbon, University Park, PA, USA, 19–23 June 1961; pp. 73–80.
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology. IUPAC Recommendations; Blackwell Science: Hoboken, NJ, USA, 1997.
- Kumar, V.; Kumar, A.; Lee, D.J.; Park, S.S. Estimation of Number of Graphene Layers Using Different Methods: A Focused Review. Materials 2021, 14, 4590.
- Kamedulski, P.; Ilnicka, A.; Lukaszewicz, J.P. Selected Aspects of Graphene Exfoliation as an Introductory Step Towards 3D Structuring of Graphene Nano-Sheets. Curr. Graphene Sci. 2019, 2, 106–117.
- Prekodravac, J.R.; Kepić, D.P.; Colmenares, J.C.; Giannakoudakis, D.A.; Jovanović, S.P. A comprehensive review on selected graphene synthesis methods: From electrochemical exfoliation through rapid thermal annealing towards biomass pyrolysis. J. Mater. Chem. C 2021, 9, 6722–6748.
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18.
- Ray, S. Applications of Graphene and Graphene-Oxide Based Nanomaterials; William Andrew: Norwich, NY, USA, 2015.
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Kim, H.S. Fast and fully–scalable synthesis of reduced graphene oxide. Sci. Rep. 2015, 5, 10160.
- Xu, Y.; Hong, W.; Bai, H.; Li, C.; Shi, G. Strong and ductile poly (vinyl alcohol)/graphene oxide composite films with a layered structure. Carbon 2009, 47, 3538–3543.
- Xu, J.; Wang, K.; Zu, S.-Z.; Han, B.-H.; Wei, Z. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 2010, 4, 5019–5026.
- Wang, L.; Ye, Y.; Lu, X.; Wen, Z.; Li, Z.; Hou, H.; Song, Y. Hierarchical nanocomposites of polyaniline nanowire arrays on reduced graphene oxide sheets for supercapacitors. Sci. Rep. 2013, 3, 3568.
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide–TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471.
- Yu, C.; Ma, P.; Zhou, X.; Wang, A.; Qian, T.; Wu, S.; Chen, Q. All-solid-state flexible supercapacitors based on highly dispersed polypyrrole nanowire and reduced graphene oxide composites. ACS Appl. Mater. Interfaces 2014, 6, 17937–17943.
- Yun, Y.J.; Hong, W.G.; Kim, W.J.; Jun, Y.; Kim, B.H. A novel method for applying reduced graphene oxide directly to electronic textiles from yarns to fabrics. Adv. Mater. 2013, 25, 5701–5705.
- Zhang, X.-J.; Wang, G.-S.; Cao, W.-Q.; Wei, Y.-Z.; Liang, J.-F.; Guo, L.; Cao, M.-S. Enhanced microwave absorption property of reduced graphene oxide (RGO)-MnFe2O4 nanocomposites and polyvinylidene fluoride. ACS Appl. Mater. Interfaces 2014, 6, 7471–7478.
- Zhou, X.; Chen, Q.; Wang, A.; Xu, J.; Wu, S.; Shen, J. Bamboo–like composites of V2O5/polyindole and activated carbon cloth as electrodes for all-solid-state flexible asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 3776–3783.
- Xu, Y.; Wang, L.; Cao, P.; Cai, C.; Fu, Y.; Ma, X. Mesoporous composite nickel cobalt oxide/graphene oxide synthesized via a template-assistant co-precipitation route as electrode material for supercapacitors. J. Power Sources 2016, 306, 742–752.
- Zhao, C.; Wang, Q.; Zhang, H.; Passerini, S.; Qian, X. Two-dimensional titanium carbide/RGO composite for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 15661–15667.
- Han, Z.J.; Seo, D.H.; Yick, S.; Chen, J.H.; Ostrikov, K.K. MnOx/carbon nanotube/reduced graphene oxide nanohybrids as high-performance supercapacitor electrodes. NPG Asia Mater. 2014, 6, e140.
- Cao, X.; Zheng, B.; Shi, W.; Yang, J.; Fan, Z.; Luo, Z.; Rui, X.; Chen, B.; Yan, Q.; Zhang, H. Reduced graphene oxide-wrapped MoO3 composites prepared by using metal–organic frameworks as precursor for all-solid-state flexible supercapacitors. Adv. Mater. 2015, 27, 4695–4701.
- Dong, X.; Wang, K.; Zhao, C.; Qian, X.; Chen, S.; Li, Z.; Liu, H.; Dou, S. Direct synthesis of RGO/Cu2O composite films on Cu foil for supercapacitors. J. Alloys Compd. 2014, 586, 745–753.
- Ghorbani, M.; Golobostanfard, M.R.; Abdizadeh, H. Flexible freestanding sandwich type ZnO/rGO/ZnO electrode for wearable supercapacitor. Appl. Surf. Sci. 2017, 419, 277–285.
- Hu, Y.; Guan, C.; Ke, Q.; Yow, Z.F.; Cheng, C.; Wang, J. Hybrid Fe2O3 nanoparticle clusters/rGO paper as an effective negative electrode for flexible supercapacitors. Chem. Mater. 2016, 28, 7296–7303.
- Ali, G.A.M.; Yusoff, M.M.; Algarni, H.; Chong, K.F. One-step electrosynthesis of MnO2/rGO nanocomposite and its enhanced electrochemical performance. Ceram. Int. 2018, 44, 7799–7807.
- Thalji, M.R.; Ali, G.A.M.; Liu, P.; Zhong, Y.L.; Chong, K.F. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem. Eng. J. 2021, 409, 128216.
- Kabiri, R.; Namazi, H. Nanocrystalline cellulose acetate (NCCA)/graphene oxide (GO) nanocomposites with enhanced mechanical properties and barrier against water vapor. Cellulose 2014, 21, 3527–3539.
- Kafy, A.; Sadasivuni, K.K.; Kim, H.-C.; Akther, A.; Kim, J. Designing flexible energy and memory storage materials using cellulose modified graphene oxide nanocomposites. Phys. Chem. Chem. Phys. 2015, 17, 5923–5931.
- Kim, H.; Cho, M.Y.; Kim, M.H.; Park, K.Y.; Gwon, H.; Lee, Y.; Roh, K.C.; Kang, K. A novel high-energy hybrid supercapacitor with an anatase TiO2-reduced graphene oxide anode and an activated carbon cathode. Adv. Energy Mater. 2013, 3, 1500–1506.
- Mianehrow, H.; Sabury, S.; Bazargan, A.; Sharif, F.; Mazinani, S. A flexible electrode based on recycled paper pulp and reduced graphene oxide composite. J. Mater. Sci. Mater. Electron. 2017, 28, 4990–4996.
This entry is offline, you can click here to edit this entry!