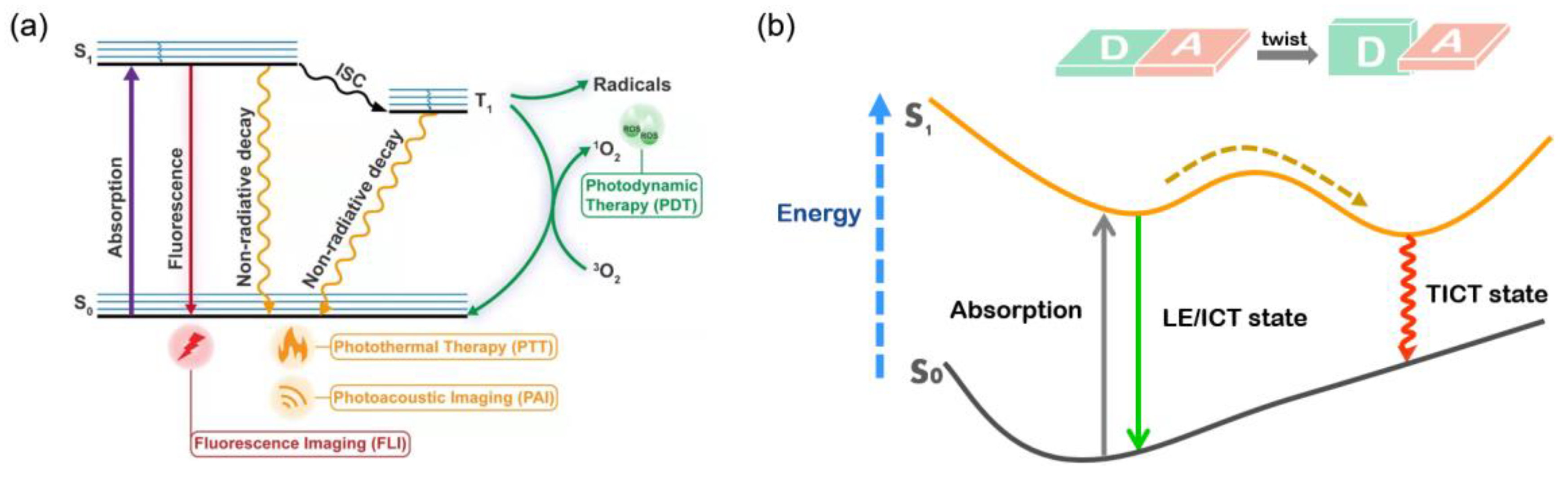
Various modalities are involved in phototheranostic systems, including therapeutic methods such as photodynamic therapy (PDT) and photothermal therapy (PTT), and diagnostic technologies such as photothermal imaging (PTI), photoacoustic imaging (PAI), and fluorescence imaging (FLI). As an emerging strategy for cancer treatments via generating reactive oxygen species (ROS) with the assistance of light, tissue oxygen, and photosensitizer (PS), PDT has a remarkable light-controllable ability, specific spatiotemporal selectivity, and minimized invasiveness. Second near-infrared (NIR-II) fluorophores possess the capability of surmounting the inherent deficiencies of conventional FLI, by virtue of its remarkable features including deep penetration, reduced tissue scattering, minimal damage, and high spatial resolution endowed by the extremely long wavelength.
- aggregation-induced emission
- NIR-II emission
- phototheranostics
- cancer treatment
1. Introduction
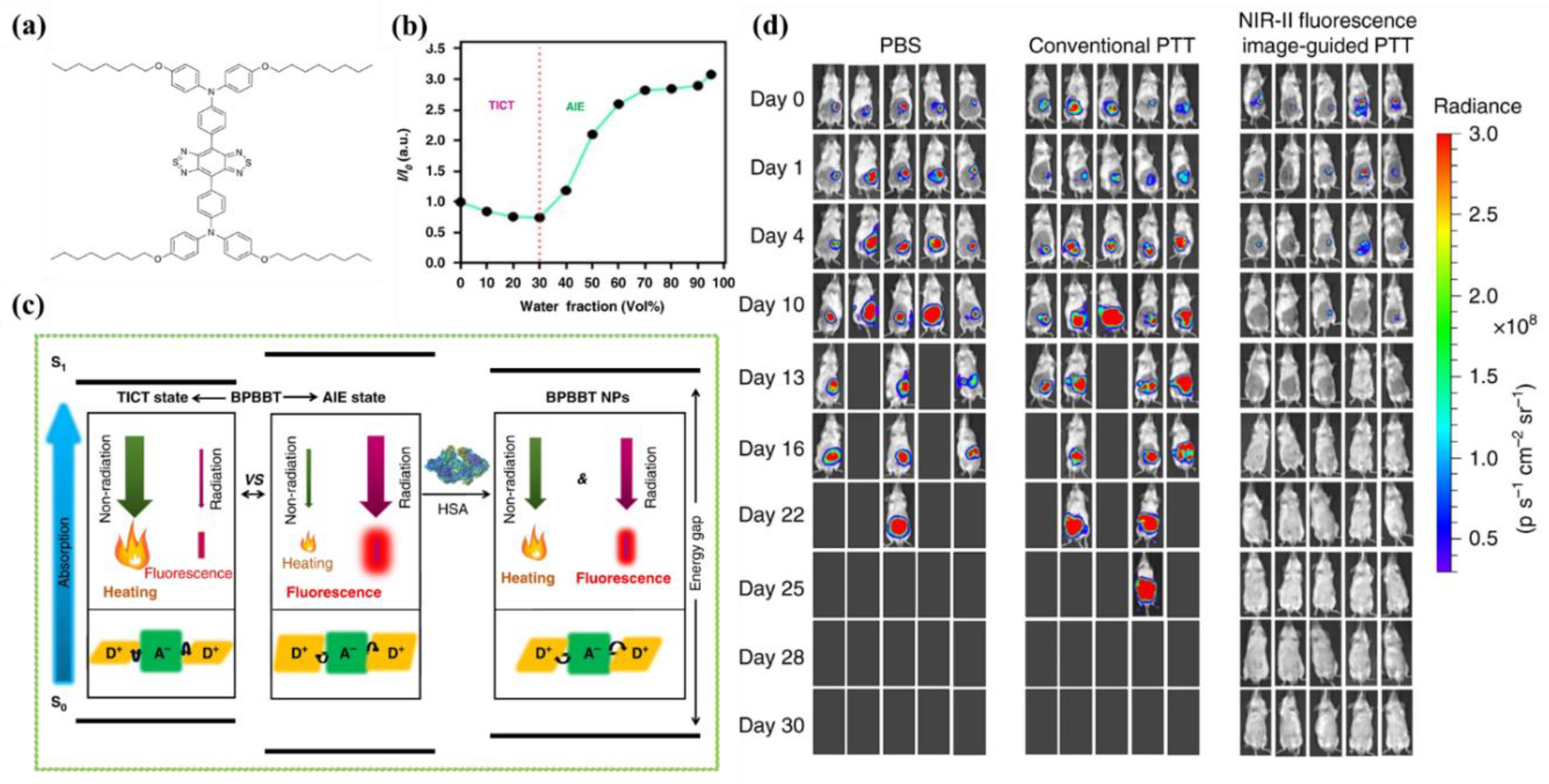
2. NIR-II FLI-Guided PTT
3. PAI-Guided PTT Based on NIR-II Fluorophores
4. Multimodal Imaging-Guided Synergistic Therapy
This entry is adapted from the peer-reviewed paper 10.3390/bios12010046
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021, 149, 778–789.
- Shi, J.J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37.
- Dierolf, M.; Menzel, A.; Thibault, P.; Schneider, P.; Kewish, C.M.; Wepf, R.; Bunk, O.; Pfeiffer, F. Ptychographic X-ray computed tomography at the nanoscale. Nature 2010, 467, 436–439.
- Gambhir, S.S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2002, 2, 683–693.
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698.
- Wang, D.; Lee, M.M.S.; Xu, W.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Theranostics based on AIEgens. Theranostics 2018, 8, 4925–4956.
- Wang, D.; Lee, M.M.S.; Xu, W.; Shan, G.; Zheng, X.; Kwok, R.T.K.; Lam, J.W.Y.; Hu, X.; Tang, B.Z. Boosting Non-Radiative Decay to Do Useful Work: Development of a Multi-Modality Theranostic System from an AIEgen. Angew. Chem. Int. Ed. 2019, 58, 5628–5632.
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677.
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519.
- Chen, C.; Ni, X.; Jia, S.; Liang, Y.; Wu, X.; Kong, D.; Ding, D. Massively Evoking Immunogenic Cell Death by Focused Mitochondrial Oxidative Stress using an AIE Luminogen with a Twisted Molecular Structure. Adv. Mater. 2019, 31, 1904914.
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Bi, R.Z.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144.
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N.; Eliceiri, K.W.; Kuo, J.S.; Weichert, J.P. Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol. 2017, 14, 347–364.
- Day, R.N.; Davidson, M.W. The fluorescent protein palette: Tools for cellular imaging. Chem. Soc. Rev. 2009, 38, 2887–2921.
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589.
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741.
- Song, N.; Zhang, Z.; Liu, P.; Yang, Y.; Wang, L.; Wang, D.; Tang, B.Z. Nanomaterials with Supramolecular Assembly Based on AIE Luminogens for Theranostic Applications. Adv. Mater. 2020, 32, 2004208.
- Kang, M.; Zhang, Z.; Song, N.; Li, M.; Sun, P.; Chen, X.; Wang, D.; Tang, B.Z. Aggregation-enhanced theranostics: AIE sparkles in biomedical field. Aggregate 2020, 1, 80–106.
- Feng, G.; Liu, B. Multifunctional AIEgens for Future Theranostics. Small 2016, 12, 6528–6535.
- Wang, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens for Activity-Based Sensing. Acc. Chem. Res. 2019, 52, 2559–2570.
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479.
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940.
- Smith, A.M.; Mancini, M.C.; Nie, S.M. Bioimaging Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711.
- Hong, G.; Lee, J.C.; Robinson, J.T.; Raaz, U.; Xie, L.; Huang, N.; Cooke, J.P.; Dai, H. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 2012, 18, 1841–1846.
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138.
- Qian, G.; Dai, B.; Luo, M.; Yu, D.; Zhan, J.; Zhang, Z.; Ma, D.; Wang, Z. Band Gap Tunable, Donor-Acceptor-Donor Charge-Transfer Heteroquinoid-Based Chromophores: Near Infrared Photoluminescence and Electroluminescence. Chem. Mater. 2008, 20, 6208–6216.
- Xie, C.; Zhou, W.; Zeng, Z.; Fan, Q.; Pu, K. Grafted semiconducting polymer amphiphiles for multimodal optical imaging and combination phototherapy. Chem. Sci. 2020, 11, 10553–10570.
- Wang, W.; He, X.; Du, M.; Xie, C.; Zhou, W.; Huang, W.; Fan, Q. Organic Fluorophores for 1064 nm Excited NIR-II Fluorescence Imaging. Front. Chem. 2021, 9, 769655.
- Shao, A.; Xie, Y.; Zhu, S.; Guo, Z.; Zhu, S.; Guo, J.; Shi, P.; James, T.; Tian, H.; Zhu, W. Far-Red and Near-IR AIE-Active Fluorescent Organic Nanoprobes with Enhanced Tumor-Targeting Efficacy: Shape-Specific Effects. Angew. Chem. Int. Ed. 2015, 54, 7275–7280.
- Liu, J.; Chen, C.; Ji, S.; Liu, Q.; Ding, D.; Zhao, D.; Liu, B. Long wavelength excitable near-infrared fluorescent nanoparticles with aggregation-induced emission characteristics for image-guided tumor resection. Chem. Sci. 2017, 8, 2782–2789.
- Li, Y.; Hu, D.; Sheng, Z.; Min, T.; Zha, M.; Ni, J.; Zheng, H.; Li, K. Self-assembled AIEgen nanoparticles for multiscale NIR-II vascular imaging. Biomaterials 2021, 264, 120365.
- Niu, G.; Zhang, R.; Shi, X.; Park, H.; Xie, S.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE luminogens as fluorescent bioprobes. Trends Anal. Chem. 2020, 123, 115769.
- Jiang, Y.; Li, J.; Zhen, X.; Xie, C.; Pu, K. Dual-Peak Absorbing Semiconducting Copolymer Nanoparticles for First and Second Near-Infrared Window Photothermal Therapy: A Comparative Study. Adv. Mater. 2018, 30, 1705980.
- Wang, K.; Zhuang, J.; Chen, L.; Xu, D.; Zhang, X.; Chen, Z.; Wei, Y.; Zhang, Y. One-pot synthesis of AIE based bismuth sulfide nanotheranostics for fluorescence imaging and photothermal therapy. Colloids Surf. B Biointerfaces 2017, 160, 297–304.
- Zhang, Z.; Kang, M.; Wang, Y.; Song, G.; Wen, H.; Wang, D.; Tang, B.Z. Recent Advances of Aggregation-induced Emission Materials in Phototheranostics. Chin. J. Lumin. 2021, 42, 361–378.
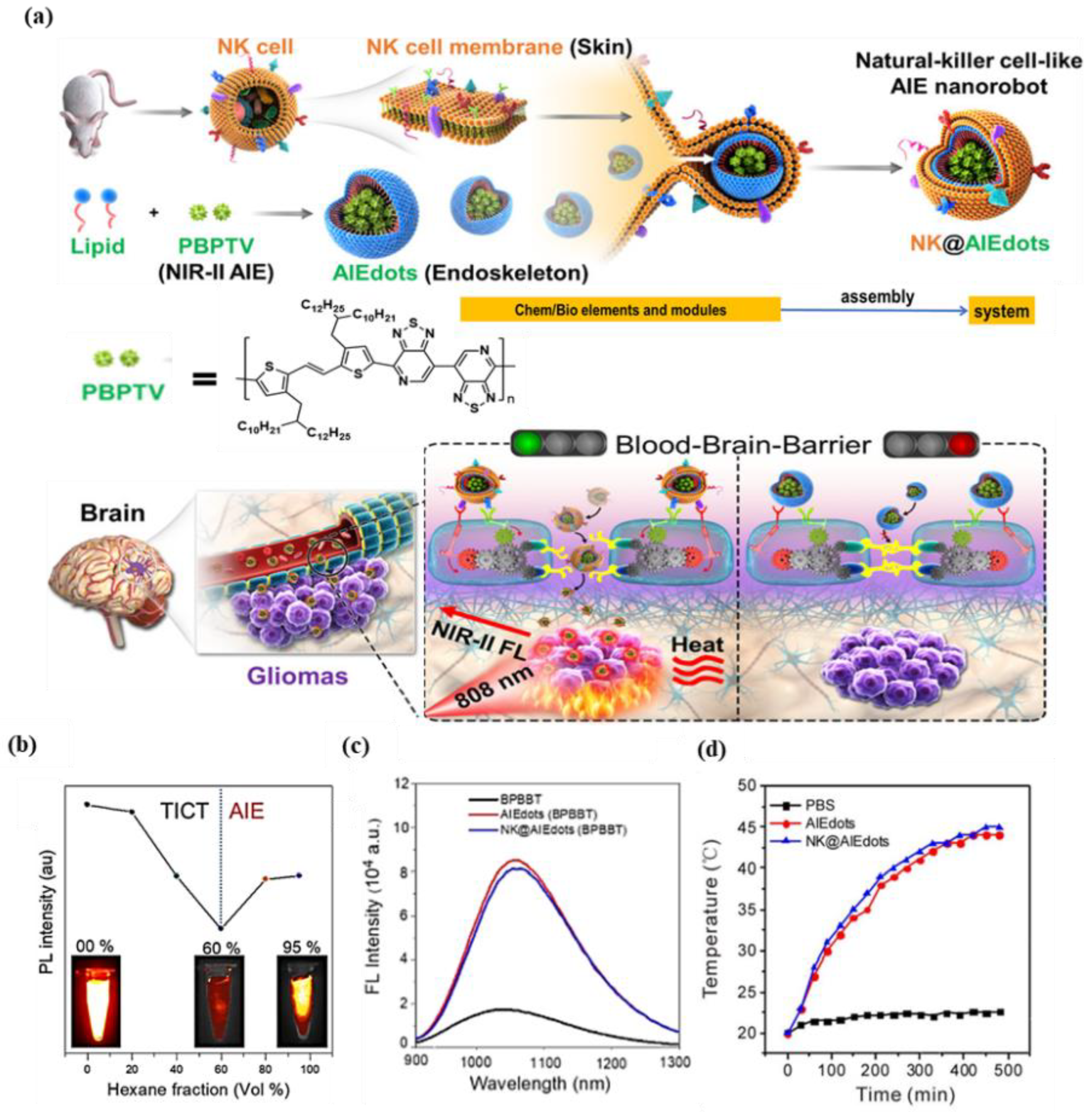
- Zhang, M.; Wang, W.; Mohammadniaei, M.; Zheng, T.; Zhang, Q.; Ashley, J.; Liu, S.; Sun, Y.; Tang, B.Z. Upregulating Aggregation-Induced-Emission Nanoparticles with Blood-Tumor-Barrier Permeability for Precise Photothermal Eradication of Brain Tumors and Induction of Local Immune Responses. Adv. Mater. 2021, 33, 2008802.
- Li, H.; Wen, H.; Zhang, Z.; Song, N.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, L.; Wang, D.; Tang, B.Z. Reverse Thinking of the Aggregation-Induced Emission Principle: Amplifying Molecular Motions to Boost Photothermal Efficiency of Nanofibers. Angew. Chem. Int. Ed. 2020, 59, 20371–20375.
- Zhang, M.; Li, J.; Yu, L.; Wang, X.; Bai, M. Tuning the fluorescence based on the combination of TICT and AIE emission of a tetraphenylethylene with D-pi-A structure. Rsc. Adv. 2020, 10, 14520–14524.
- Wang, D.; Chen, L.; Zhao, X.; Yan, X. Enhancing near-infrared AIE of photosensitizer with twisted intramolecular charge transfer characteristics via rotor effect for AIE imaging-guided photodynamic ablation of cancer cells. Talanta 2021, 225, 122046.
- Wang, C.; Chi, W.; Qiao, Q.; Tan, D.; Xu, Z.; Liu, X. Twisted intramolecular charge transfer (TICT) and twists beyond TICT: From mechanisms to rational designs of bright and sensitive fluorophores. Chem. Soc. Rev. 2021, 50, 12656–12678.
- Hu, R.; Lager, E.; Aguilar-Aguilar, A.; Liu, J.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Zhong, Y.; Wong, K.; Pena-Cabrera, E.; et al. Twisted Intramolecular Charge Transfer and Aggregation-Induced Emission of BODIPY Derivatives. J. Phys. Chem. C 2009, 113, 15845–15853.
- Yang, Q.; Ma, Z.; Wang, H.; Zhou, B.; Zhu, S.J.; Zhong, Y.; Wang, J.Y.; Wan, H.; Antaris, A.; Ma, R.; et al. Rational Design of Molecular Fluorophores for Biological Imaging in the NIR-II Window. Adv. Mater. 2017, 29, 1605497.
- Morris, W.A.; Kolpaczynska, M.; Fraser, C.L. Effects of alpha-Substitution on Mechanochromic Luminescence and Aggregation-Induced Emission of Difluoroboron beta-Diketonate Dyes. J. Phys. Chem. C 2016, 120, 22539–22548.
- Ge, S.; Li, B.; Meng, X.; Yan, H.; Yang, M.; Dong, B.; Lu, Y. Aggregation-induced emission, multiple chromisms and self organization of N-substituted-1,8-naphthalimides. Dyes Pigm. 2018, 148, 147–153.
- Cai, Y.; Liang, P.; Tang, Q.; Yang, X.; Si, W.; Huang, W.; Zhang, Q.; Dong, X. Diketopyrrolopyrrole-Triphenylamine Organic Nanoparticles as Multifunctional Reagents for Photoacoustic Imaging-Guided Photodynamic/Photothermal Synergistic Tumor Therapy. ACS Nano 2017, 11, 1054–1063.
- Wang, Y.; Zhu, W.; Du, W.; Liu, X.; Zhang, X.; Dong, H.; Hu, W. Cocrystals Strategy towards Materials for Near-Infrared Photothermal Conversion and Imaging. Angew. Chem. Int. Ed. 2018, 57, 3963–3967.
- Gao, S.; Wei, G.; Zhang, S.; Zheng, B.; Xu, J.; Chen, G.; Li, M.; Song, S.; Fu, W.; Xiao, Z.; et al. Albumin tailoring fluorescence and photothermal conversion effect of near-infrared-II fluorophore with aggregation-induced emission characteristics. Nat. Commun. 2019, 10, 2206.
- Wang, H.; Mu, X.; Yang, J.; Liang, Y.; Zhang, X.; Ming, D. Brain imaging with near-infrared fluorophores. Coord. Chem. Rev. 2019, 380, 550–571.
- Deng, G.; Peng, X.; Sun, Z.; Zheng, W.; Yu, J.; Du, L.; Chen, H.; Gong, P.; Zhang, P.; Cai, L.; et al. Natural-Killer-Cell-Inspired Nanorobots with Aggregation-Induced Emission Characteristics for Near-Infrared-II Fluorescence-Guided Glioma Theranostics. ACS Nano 2020, 14, 11452–11462.
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688.
- Liu, S.; Zhou, X.; Zhang, H.; Ou, H.; Lam, J.W.Y.; Liu, Y.; Shi, L.; Ding, D.; Tang, B.Z. Molecular Motion in Aggregates: Manipulating TICT for Boosting Photothermal Theranostics. J. Am. Chem. Soc. 2019, 141, 5359–5368.
- Qi, J.; Duan, X.; Cai, Y.; Jia, S.; Chen, C.; Zhao, Z.; Li, Y.; Peng, H.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Simultaneously boosting the conjugation, brightness and solubility of organic fluorophores by using AIEgens. Chem. Sci. 2020, 11, 8438–8447.
- Chen, J.; Qi, J.; Chen, C.; Chen, J.; Liu, L.; Gao, R.; Zhang, T.; Song, L.; Ding, D.; Zhang, P.; et al. Tocilizumab-Conjugated Polymer Nanoparticles for NIR-II Photoacoustic-Imaging-Guided Therapy of Rheumatoid Arthritis. Adv. Mater. 2020, 32, 2003399.
- Wang, L.H.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462.
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.R.; Ma, T.J.; Oralkan, O.; et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008, 3, 557–562.
- Xu, M.H.; Wang, L.H.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101.
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2019, 31, 1805875.
- Zhou, Y.; Wang, D.; Zhang, Y.; Chitgupi, U.; Geng, J.; Wang, Y.; Zhang, Y.; Cook, T.R.; Xia, J.; Lovell, J.F. A Phosphorus Phthalocyanine Formulation with Intense Absorbance at 1000 nm for Deep Optical Imaging. Theranostics 2016, 6, 688–697.
- Wu, J.Y.Z.; You, L.Y.; Lan, L.; Lee, H.J.; Chaudhry, S.T.; Li, R.; Cheng, J.X.; Mei, J.G. Semiconducting Polymer Nanoparticles for Centimeters-Deep Photoacoustic Imaging in the Second Near-Infrared Window. Adv. Mater. 2017, 29, 1703403.
- Aoki, H.; Nojiri, M.; Mukai, R.; Ito, S. Near-infrared absorbing polymer nano-particle as a sensitive contrast agent for photo-acoustic imaging. Nanoscale 2015, 7, 337–343.
- Guo, X.; Cao, B.; Wang, C.; Lu, S.; Hu, X. In vivo photothermal inhibition of methicillin-resistant Staphylococcus aureus infection by in situ templated formulation of pathogen-targeting phototheranostics. Nanoscale 2020, 12, 7651–7659.
- Qi, J.; Fang, Y.; Kwok, R.T.K.; Zhang, X.; Hu, X.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Highly Stable Organic Small Molecular Nanoparticles as an Advanced and Biocompatible Phototheranostic Agent of Tumor in Living Mice. ACS Nano 2017, 11, 7177–7188.
- Song, S.; Zhao, Y.; Kang, M.; Zhang, Z.; Wu, Q.; Fu, S.; Li, Y.; Wen, H.; Wang, D.; Tang, B.Z. Side-Chain Engineering of Aggregation-Induced Emission Molecules for Boosting Cancer Phototheranostics. Adv. Funct. Mater. 2021.
- Gao, H.; Cheng, T.; Liu, J.; Liu, J.; Yang, C.; Chu, L.; Zhang, Y.; Ma, R.; Shi, L. Self-Regulated Multifunctional Collaboration of Targeted Nanocarriers for Enhanced Tumor Therapy. Biomacromolecules 2014, 15, 3634–3642.
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986.
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650.
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640.
- Zhao, X.; Long, S.; Li, M.; Cao, J.; Li, Y.; Guo, L.; Sun, W.; Du, J.; Fan, J.; Peng, X. Oxygen-Dependent Regulation of Excited-State Deactivation Process of Rational Photosensitizer for Smart Phototherapy. J. Am. Chem. Soc. 2020, 142, 1510–1517.
- Yan, D.; Xie, W.; Zhang, J.; Wang, L.; Wang, D.; Tang, B.Z. Donor/pi-Bridge Manipulation for Constructing a Stable NIR-II Aggregation-Induced Emission Luminogen with Balanced Phototheranostic Performance. Angew. Chem. Int. Ed. 2021, 133, 26973–26980.
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885.
