Scoliosis is defined as a three-dimensional spinal deformity consisting of a lateral curvature greater than 10 degrees with rotation of the vertebrae within the curve. It can be identified as congenital, neuromuscular or idiopathic. Idiopathic scoliosis (IS) can be further classified by age of onset: infantile (birth to two years), juvenile (three to nine years), and adolescent (10 years and older). It is the most common pediatric musculoskeletal disorder that causes a three-dimensional (3D) spinal deformity. The deformity is always 3D because it also involves an axial rotation of the vertebrae, not just displacement and rotation in the frontal plane. Adolescent IS is the most common form because the spinal deformity evolves during periods of significant physical growth. IS is diagnosed when other etiological factors cannot be identified, such as congenital neurological or musculoskeletal anomalies, or inflammatory or demyelinating processes leading to primary or secondary motor neuron damage (myotonia, myopathy, etc.).
- spine
- diagnostic imaging
- computer analysis
- artificial intelligence diagnosis
- scoliosis
- spinal curvatures
1. Methods for Imaging and Evaluation of Scoliosis Using Radiography
2. Method for Imaging and Evaluation of Scoliosis Using Magnetic Resonance Imaging (MRI)
3. Computed Tomography (CT)
4. Artificial Intelligence (AI) As a Method for Detection of Scoliosis
-
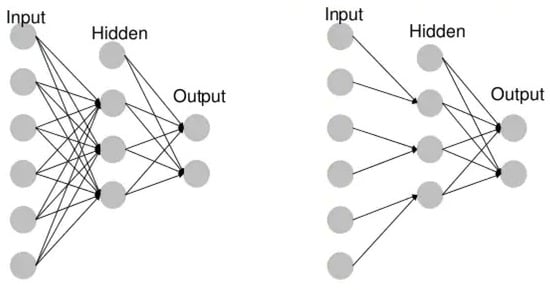
Unidirectional neural networks, i.e., multilayer perceptrons (MLP) (Figure 1), consist of an input layer, a hidden layer(s) and an output layer. While these neural networks are also commonly referred to as MLPs, keep in mind that they are actually sigmoidal neurons, not perceptrons, as most real-world problems are non-linear. Data are used to train these models. They form the basis of computer vision, natural language processing and other neural networks.
-
Convolutional neural networks (CNNs) are similar to unidirectional networks but are typically used for image recognition, pattern recognition and/or computer vision. These networks use the principles of linear algebra, in particular, matrix multiplication, to identify patterns in an image.
-
Recursive neural networks (RNNs) are distinguished based on feedback loops.
4.1. Neural Networks and Deep Learning
4.2. Automatic Measurement Algorithm of Scoliosis Cobb Angle Based on Deep Learning
This entry is adapted from the peer-reviewed paper 10.3390/s21248410
References
- Kalender, W.A. X-ray Computed Tomography. Phys. Med. Biol. 2006, 51, R29–R43.
- Hoffman, D.A.; Lonstein, J.E.; Morin, M.M.; Visscher, W.; Harris, B.S.; Boice, J.D. Breast Cancer in Women with Scoliosis Exposed to Multiple Diagnostic x Rays. J. Natl. Cancer Inst. 1989, 81, 1307–1312.
- Kutanzi, K.; Lumen, A.; Koturbash, I.; Miousse, I. Pediatric Exposures to Ionizing Radiation: Carcinogenic Considerations. Int. J. Environ. Res. Public Health 2016, 13, 1057.
- IARC Monographs on the Evaluation of Carcionogenic Risks to Humans. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/08/14-002.pdf (accessed on 1 December 2021).
- UNSCEAR 2013 Report to the General Assembly with Scientific Annexes Volume II: Scientific Annex B. 283. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/UNSCEAR_2013_Report_Annex_B_Children.pdf (accessed on 1 December 2021).
- Harzmann, H.C. Optischer Gipsabdruck Hilft Bei Der Rückenanalyse. Süddeutscher Orthopädenkongress Kongressausgabe 1999, 2, 15.
- Harzmann, H.C. Stellenwert Der Videorasterstereographie Als Schulärztliche Screeningmethode von Skoliotischen Fehlhaltungen Und Strukturellen Skoliosen. PhD Thesis, Ludwig-Maximilians-Universität, Medizinischen Fakultät, München, Germany, 2000.
- Betsch, M.; Wild, M.; Jungbluth, P.; Thelen, S.; Hakimi, M.; Windolf, J.; Horstmann, T.; Rapp, W. The Rasterstereographic-Dynamic Analysis of Posture in Adolescents Using a Modified Matthiass Test. Eur. Spine J. 2010, 19, 1735–1739.
- Coblentz, A.M.; Herron, R.E. (Eds.) NATO Symposium on Applications of Human Biostereoietrics; Proceedings Volume; Society of Photo-Optical Instrumentation Engineers: Paris, France, 1980; Volume 166.
- Frobin, W. Rasterstereography: A Photogrammetric Method for Measurement of Body Surfaces. Photogramm. Eng. Remote. Sens. 1981, 47, 1717–1724.
- Hackenberg, L.; Hierholzer, E.; Pötzl, W.; Götze, C.; Liljenqvist, U. Rasterstereographic Back Shape Analysis in Idiopathic Scoliosis after Posterior Correction and Fusion. Clin. Biomech. 2003, 18, 883–889.
- Mangone, M.; Raimondi, P.; Paoloni, M.; Pellanera, S.; Di Michele, A.; Di Renzo, S.; Vanadia, M.; Dimaggio, M.; Murgia, M.; Santilli, V. Vertebral Rotation in Adolescent Idiopathic Scoliosis Calculated by Radiograph and Back Surface Analysis-Based Methods: Correlation between the Raimondi Method and Rasterstereography. Eur. Spine J. 2013, 22, 367–371.
- Schröder, J.; Schaar, H.; Korn, M.; Färber, I.; Ziegler, M.; Braumann, K.M.; Reer, R.; Mattes, K. Zur Sensitivität Und Reproduzierbarkeit Der Pedobarographie Mit Dem System PedoScan. Deutsche Zeitschrift Sportmedizin 2007, 58, 207.
- Seiberlich, N.; Gulani, V.; Campbell, A.; Sourbron, S.; Doneva, M.I.; Calamante, F.; Hu, H.H. Quantitative Magnetic Resonance Imaging; Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-12-817058-8.
- Yetişir, F. Local and Global SAR Constrained Large Tip Angle 3D KT-Points Parallel Transmit Pulse Design at 7 T, Massachusetts Institute of Technology: Massachusetts, 2014. Available online: https://dspace.mit.edu/handle/1721.1/87790 (accessed on 1 December 2021).
- Davids, J.R.; Chamberlin, E.; Blackhurst, D.W. Indications for Magnetic Resonance Imaging in Presumed Adolescent Idiopathic Scoliosis. J. Bone Jt. Surg. 2004, 86, 2187–2195.
- Maiocco, B.; Deeney, V.F.; Coulon, R.; Parks, P.F. Adolescent Idiopathic Scoliosis and the Presence of Spinal Cord Abnormalities. Spine 1997, 22, 2537–2541.
- Do, T.M.D.; Fras, C.M.D.; Burke, S.M.D.; Widmann, R.F.M.D.; Rawlins, B.M.D.; Boachie-Adjei, O.M.D. Clinical Value of Routine Preoperative Magnetic Resonance Imaging in Adolescent Idiopathic Scoliosis. J. Bone Jt. Surg. 2001, 83, 577–579.
- Wright, N. Imaging in Scoliosis. Arch. Dis. Child. 2000, 82, 38–40.
- Zhu, C.; Huang, S.; Song, Y.; Liu, H.; Liu, L.; Yang, X.; Zhou, C.; Hu, B.; Chen, H. Surgical Treatment of Scoliosis-Associated with Syringomyelia: The Role of Syrinx Size. Neurol. India 2020, 68, 299.
- Shih, R.Y.; Koeller, K.K. Intramedullary Masses of the Spinal Cord: Radiologic-Pathologic Correlation. RadioGraphics 2020, 40, 1125–1145.
- Bolognese, P.A.; Brodbelt, A.; Bloom, A.B.; Kula, R.W. Chiari I Malformation: Opinions on Diagnostic Trends and Controversies from a Panel of 63 International Experts. World Neurosurg. 2019, 130, e9–e16.
- Rogers, B.P.; Haughton, V.M.; Arfanakis, K.; Meyerand, M.E. Application of Image Registration to Measurement of Intervertebral Rotation in the Lumbar Spine. Magn. Reson. Med. 2002, 48, 1072–1075.
- Rogers, B.; Wiese, S.; Blankenbaker, D.; Meyerand, E.; Haughton, V. Accuracy of an Automated Method to Measure Rotations of Vertebrae from Computerized Tomography Data. Spine 2005, 30, 694–696.
- Carro, P.A. Magnetic Resonance Imaging in Children with Scoliosis. Semin. Musculoskelet. Radiol. 1999, 3, 257–266.
- Heinrich, A.; Reinhold, M.; Güttler, F.V.; Matziolis, G.; Teichgräber, U.K.-M.; Zippelius, T.; Strube, P. MRI Following Scoliosis Surgery? An Analysis of Implant Heating, Displacement, Torque, and Susceptibility Artifacts. Eur. Radiol. 2021, 31, 4298–4307.
- Ahmad, F.U.; Sidani, C.; Fourzali, R.; Wang, M.Y. Postoperative Magnetic Resonance Imaging Artifact with Cobalt-Chromium versus Titanium Spinal Instrumentation. J. Neurosurg. Spine 2013, 19, 629–636.
- Etemadifar, M.R.; Andalib, A.; Rahimian, A.; Nodushan, S.M.H.T. Cobalt Chromium-Titanium Rods versus Titanium-Titanium Rods for Treatment of Adolescent Idiopathic Scoliosis; Which Type of Rod Has Better Postoperative Outcomes? Rev. Assoc. Med. Bras. 2018, 64, 1085–1090.
- Buzug, T.M. Computed Tomography. In Springer Handbook of Medical Technology; Krame, R., Hoffmann, K.P., Pozos, R.S., Eds.; Springer Handbooks; Springer: Berlin/Heidelberg, Germany, 2011.
- Brenner, D.J.; Hall, E.J. Computed Tomography--an Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284.
- Yawn, B.P.; Yawn, R.A.; Hodge, D.; Kurland, M.; Shaughnessy, W.J.; Ilstrup, D.; Jacobsen, S.J. A Population-Based Study of School Scoliosis Screening. JAMA 1999, 282, 1427–1432.
- Chen, K.; Zhai, X.; Sun, K.; Wang, H.; Yang, C.; Li, M. A Narrative Review of Machine Learning as Promising Revolution in Clinical Practice of Scoliosis. Ann. Transl. Med. 2021, 9, 67.
- Yang, J.; Zhang, K.; Fan, H.; Huang, Z.; Xiang, Y.; Yang, J.; He, L.; Zhang, L.; Yang, Y.; Li, R.; et al. Development and Validation of Deep Learning Algorithms for Scoliosis Screening Using Back Images. Commun. Biol. 2019, 2, 390.
- Pazos, V.; Cheriet, F.; Song, L.; Labelle, H.; Dansereau, J. Accuracy Assessment of Human Trunk Surface 3D Reconstructions from an Optical Digitising System. Med. Biol. Eng. Comput. 2005, 43, 11–15.
- Jaremko, J.L.; Poncet, P.; Ronsky, J.; Harder, J.; Dansereau, J.; Labelle, H.; Zernicke, R.F. Estimation of Spinal Deformity in Scoliosis From Torso Surface Cross Sections. Spine 2001, 26, 1583–1591.
- Komeili, A.; Westover, L.; Parent, E.C.; El-Rich, M.; Adeeb, S. Correlation Between a Novel Surface Topography Asymmetry Analysis and Radiographic Data in Scoliosis. Spine Deform. 2015, 3, 303–311.
- Ramirez, L.; Durdle, N.G.; Raso, V.J.; Hill, D.L. A Support Vector Machines Classifier to Assess the Severity of Idiopathic Scoliosis From Surface Topography. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 84–91.
- Bergeron, C.; Cheriet, F.; Ronsky, J.; Zernicke, R.; Labelle, H. Prediction of Anterior Scoliotic Spinal Curve from Trunk Surface Using Support Vector Regression. Eng. Appl. Artif. Intell. 2005, 18, 973–983.
- Liu, X.C.; Thometz, J.G.; Lyon, R.M.; Klein, J. Functional Classification of Patients with Idiopathic Scoliosis Assessed by the Quantec System. Spine 2001, 26, 1274–1279.
- Zhang, J.; Lou, E.; Hill, D.L.; Raso, J.V.; Wang, Y.; Le, L.H.; Shi, X. Computer-Aided Assessment of Scoliosis on Posteroanterior Radiographs. Med. Biol. Eng. Comput. 2010, 48, 185–195.
- Samuvel, B.; Thomas, V.; Mini, M.G.; Kumar, J.R. A Mask Based Segmentation Algorithm for Automatic Measurement of Cobb Angle from Scoliosis X-ray Image. In Proceedings of the 2012 International Conference on Advances in Computing and Communications, Cochin, India, 9–11 August 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 110–113.
- Zhang, J.; Li, H.; Lv, L.; Zhang, Y. Computer-Aided Cobb Measurement Based on Automatic Detection of Vertebral Slopes Using Deep Neural Network. Int. J. Biomed. Imaging 2017, 2017, 9083916.
- Wu, W.; Liang, J.; Du, Y.; Tan, X.; Xiang, X.; Wang, W.; Ru, N.; Le, J. Reliability and Reproducibility Analysis of the Cobb Angle and Assessing Sagittal Plane by Computer-Assisted and Manual Measurement Tools. BMC Musculoskelet. Disord. 2014, 15, 33.
- Shaw, M.; Adam, C.J.; Izatt, M.T.; Licina, P.; Askin, G.N. Use of the IPhone for Cobb Angle Measurement in Scoliosis. Eur. Spine J. 2012, 21, 1062–1068.
- Dollár, P.; Appel, R.; Belongie, S.; Perona, P. Fast Feature Pyramids for Object Detection. IEEE Trans. Pattern Anal. Mach. Intell. 2014, 36, 1532–1545.
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation; Springer: Berlin/Heidelberg, Germany, 2015.
- Yongcheng, T.; Nian, W.; Fei, T.; Hemu, C. Automatic Measurement Algorithm of Scoliosis Cobb Angle Based on Deep Learning. J. Phys. Conf. Ser. 2019, 1187, 042100.
- Forsberg, D.; Lundström, C.; Andersson, M.; Vavruch, L.; Tropp, H.; Knutsson, H. Fully Automatic Measurements of Axial Vertebral Rotation for Assessment of Spinal Deformity in Idiopathic Scoliosis. Phys. Med. Biol. 2013, 58, 1775–1787.
- Anitha, H.; Prabhu, G.; Karunakar, A.K. Reliable and Reproducible Classification System for Scoliotic Radiograph Using Image Processing Techniques. J. Med. Syst. 2014, 38, 124.
- Anitha, H.; Prabhu, G.K. Automatic Quantification of Spinal Curvature in Scoliotic Radiograph Using Image Processing. J. Med. Syst. 2012, 36, 1943–1951.
- Sardjono, T.A.; Wilkinson, M.H.F.; Veldhuizen, A.G.; van Ooijen, P.M.A.; Purnama, K.E.; Verkerke, G.J. Automatic Cobb Angle Determination from Radiographic Images. Spine 2013, 38, E1256–E1262.
- Sun, H.; Zhen, X.; Bailey, C.; Rasoulinejad, P.; Yin, Y.; Li, S. Direct Estimation of Spinal Cobb Angles by Structured Multi-Output Regression. In Proceedings of the International Conference on Information Processing in Medical Imaging, Boone, IA, USA, 25–30 June 2017.
- Xue, W.; Islam, A.; Bhaduri, M.; Li, S. Direct Multitype Cardiac Indices Estimation via Joint Representation and Regression Learning. IEEE Trans. Med. Imaging 2017, 36, 2057–2067.
- Wu, H.; Bailey, C.; Rasoulinejad, P.; Li, S. Automated Comprehensive Adolescent Idiopathic Scoliosis Assessment Using MVC-Net. Med. Image Anal. 2018, 48, 1–11.
- Wang, L.; Xu, Q.; Leung, S.; Chung, J.; Chen, B.; Li, S. Accurate Automated Cobb Angles Estimation Using Multi-View Extrapolation Net.Medical Image Analysis. Med. Image Anal. 2019, 58, 101542.
- Watanabe, K.; Aoki, Y.; Matsumoto, M. An Application of Artificial Intelligence to Diagnostic Imaging of Spine Disease: Estimating Spinal Alignment from Moiré Images. Neurospine 2019, 16, 697–702.
- Choi, R.; Watanabe, K.; Jinguji, H.; Fujita, N.; Ogura, Y.; Demura, S.; Kotani, T.; Wada, K.; Miyazaki, M.; Shigematsu, H.; et al. CNN-Based Spine and Cobb Angle Estimator Using Moire Images. J. Med. Syst. 2017, 5, 135–144.
- Choi, R.; Watanabe, K.; Fujita, N.; Ogura, Y.; Matsumoto, M.; Demura, S.; Kotani, T.; Wada, K.; Miyazaki, M.; Shigematsu, H.; et al. Measurement of Vertebral Rotation from Moire Image for Screening of Adolescent Idiopathic Scoliosis. IEEE Trans. Image Electron. Vis. Comput. 2018, 6, 55–64.
- Kokabu, T.; Kanai, S.; Kawakami, N.; Uno, K.; Kotani, T.; Suzuki, T.; Tachi, H.; Abe, Y.; Iwasaki, N.; Sudo, H. An Algorithm for Using Deep Learning Convolutional Neural Networks with Three Dimensional Depth Sensor Imaging in Scoliosis Detection. Spine J. 2021, 21, 980–987.
- Sudo, H.; Kokabu, T.; Abe, Y.; Iwata, A.; Yamada, K.; Ito, Y.M.; Iwasaki, N.; Kanai, S. Automated Noninvasive Detection of Idiopathic Scoliosis in Children and Adolescents: A Principle Validation Study. Sci. Rep. 2018, 8, 17714.
