Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Orthopedics
Human bone marrow (BM) has been highlighted as a promising source of mesenchymal stromal cells (MSCs) containing various growth factors and cytokines that can be potentially utilized in regenerative procedures involving cartilage and bone.
- bone marrow
- bone marrow aspirate concentrate
- mesenchymal stromal cells
1. Introduction
Human bone marrow (BM) has been spotlighted as a promising source of mesenchymal stromal cells (MSCs) containing various growth factors and cytokines that are potentially utilized towards regenerative procedures involving cartilage and bone [1]. Following the harvesting and isolation process of the BM aspirate, the proportion of MSCs is only around 0.001% to 0.01% of the nucleated cell content of the bone marrow aspiration concentrate (BMAC) [2][3]. Hence, the quality and composition of the BM aspirate used in the preparation of the BMAC remain important to obtain optimal results upon its use in various regenerative procedures. Although the composition of the BMAC is largely dependent on the biological attributes of the patient concerned [4][5], the procedure variables such as location and technique of harvesting have a role in contributing to the variability in the yield of MSCs [6]. There is a wide range of devices and systems available to harvest and process BM aspirate, each using slightly different methods. However, the separation is usually based on the density gradient existing between the blood cells, platelets, nucleated cells, and serum proteins [7]. Based on the processing methods, the cellular and chemical composition of the BMAC may be altered, thereby leading to significant variation in their regenerative potential [8].
In BMAC, MSCs have the capacity to rebuild tissue by differentiating or inducing differentiation of native progenitors into a variety of cell types such as fibroblasts, chondroblasts, myocytes, and other forms of tissue-regenerating cells. BM also contains other multipotent cells, such as hematopoietic stem cells and vascular progenitors, which are likely to play a substantial role in the repair of damaged tissues. Various forms of clinical evidence exist to demonstrate the safety and efficacy of bone marrow-derived MSCs in an osteoarthritic (OA) knee [9][10][11][12][13]. The application of BMAC has been expanded to other indications such as a partial tear of ACL [14], meniscus injuries [15], tendon pathologies [16], bone defect in the form of delayed or non-union of fractures [17][18], chondral and osteochondral defects [19], and patellofemoral arthritis [3]. The components of BMAC are a significant amount of growth factors and cytokines in addition to MSCs and other multipotent cells. BMAC can be administered in isolation or as combination therapies in conjunction with platelet-rich plasma (PRP) [20], stromal vascular fraction (SVF) [21], or surgical procedures like core decompression [22], stress-relieving osteotomies [23], autologous chondrocyte implantation (ACI) [24], matrix-induced chondrocyte implantation (MACI) [24], and osteochondral autograft transfer system (OATS) [25]. Being a minimally invasive procedure, it is tolerated well by patients with better compliance with the post-procedural rehabilitation program. The short- and long-term results of BMAC in cartilage regeneration are encouraging with good to excellent clinical, functional, radiological outcomes [10][12][13]. No major adverse effects were reported with BMAC therapy except for donor site pain [26].
A fresh, uncultured, and unreduced volume of autologous BMAC injectate containing stromal cells are the potential regenerative and proliferative elements due to the synergistic coordination between the cellular elements and the pool of extracellular matrix, growth factors, and cytokines [27][28]. The modality of BMAC application can be given in the form of either intra-articular, intra-osseous, subchondral injections or surgical implantation with a bio-scaffold [29][30]. Among all the forms of injection, intra-articular modality remains relatively simple, easy to perform under sterile conditions whereas intra-osseous and subchondral injections require hospitalization as a daycare procedure. Due to the difficulty in accurate delivery of stromal cells into the lesion, engineered chondrogenesis came into existence which delivers a stable construct of stromal cells loaded along with bio-scaffold and growth factors [31]. Such a bio-scaffold reduces the chondrocyte loss, maintains the equal distribution of cellular structure, and enhances chondrogenesis [32]. The most commonly used bio-scaffold in the published literature are tricalcium phosphate, hyaluronate, collagen derivatives, agarose, fibrin glue, and chitosan [33][34]. Additive technology of autologous PRP or allogenic homologous platelet lysate has been admixed with engineered chondrogenesis technology to improve the clinical and functional outcome in OA knee individuals [35][36][37][38][39][40].
Several studies with the usage of BMAC for focal cartilage defects and OA knees have reported favorable outcomes. A single intra-articular BMAC injection was found to be a safe and reliable treatment option for grade 3 and 4 OA knees at 30 months follow-up [9]. Keeling et al. demonstrated improvement in pain and patient-reported outcomes in OA knee patients with BMAC injections at short- to mid-term follow-up. In severe degenerative arthritis, BMAC has shown clinical benefits compared to PRP and hyaluronate [12]. In a 5-year follow-up study, intra-articular BMAC injection has proven clinical benefits in K-L grade 1 and 2 osteoarthritis knees [41]. When admixed with an adipose tissue graft, BMAC has not shown superior results in OA knee individuals compared to BMAC alone [21]. In elderly individuals with OA knees, intra-articular BMAC has the potential to slow the timing for the arthroplasty procedure [42]. Gobbi et al.; demonstrated the complete healing of grade 4 multiple chondral injuries of the knee treated with autologous BMAC admixed with either collagen type 1 or 3. In second-look arthroscopy, hyaline-like cartilaginous tissues were demonstrated. These patients have shown no adverse events in a long-term follow-up [43]. Enea et al., have shown that scaffold-based BMAC along with microfracture to be a single-stage technique for the focal chondral defects of the knee [44]. With the MRI evidence, Krych et al. demonstrated that demineralized bone graft (DBG) admixed with BMAC improved cartilage filling in the focal cartilage defects when compared with DBG admixed with autologous PRP [45]. Conversely, a few studies have shown no significant benefits with BMAC when compared with either saline, placebo, hyaluronate, PRP, SVF, or clinical grade MSCs. No statistically significant difference was observed with autologous BMAC in terms of pain relief and functional improvement in patients with bilateral OA knee when compared with saline injections yet they claimed that BMAC was a viable cellular product for pain relief in a short term follow-up of 6 months [46].
2. Variables in the Harvesting and Processing Technique of Bone Marrow Aspiration Concentrate (BMAC)
Researchers aim to discuss the variables in the harvesting and processing technique of BMAC as shown in Figure 1 and their impact on the yield of MSCs in the final concentrate obtained.
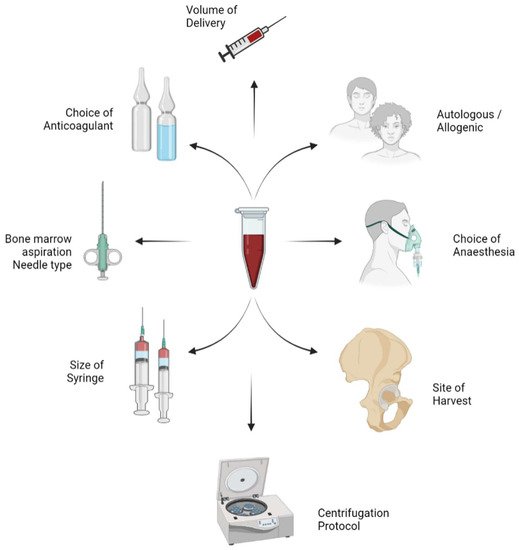
Figure 1. Variables in the harvesting and process technique of bone marrow aspiration concentrate (BMAC).
2.1. Autologous versus Allogenic Mesenchymal Stromal Cells (MSCs)
The efficacy of BMAC in cartilage regeneration depends upon the ability of the cells in the BMAC to withstand and restore the biochemical disharmony in the pathological milieu being evaluated [47][48]. Autologous sources of MSCs from bone marrow are relatively easy to harvest, cost-effective without the risk of any graft rejection or disease transmission [49]. However, autologous MSC products need a two-staged procedure for cartilage regeneration while planning for culture expansion whereas allogeneic MSC preparations can be delivered as a single staged procedure with the desired dosage of MSCs [50]. Allogeneic products namely CARTISTEM (allogeneic cord blood-derived MSCs—2.5 × 106 cells/500 μL/cm2 area of knee cartilage) [51], Stempeucell (allogeneic ex-vivo cultured pooled human BM-MSCs—2 × 108 cells cryopreserved and stored in 15 mL cryo-bags) [52], and JointStem (autologous AD-MSCs—10 × 107 cells) [53] launched the MSC-derived products with a definite dosage for cartilage injuries. Nonetheless, allogeneic MSC preparation lacks literature evidence in terms of long-term safety, and efficacy [54]. The dynamic fate of implanted allogeneic MSCs is under debate.
Due to cellular heterogeneity and the subjective characteristics of the MSCs harvested, autologous sources of the MSC cocktail generate inconsistent results upon the analysis in varied scenarios whereas the allogeneic pool of MSC contains homogeneous cells with theoretical grounds to deliver consistent results although they also suffer from immune reactions [54][55][56]. Hence, the clinical outcome depends on the harvesting and cultural characteristics of MSCs isolated either from the autologous or allogeneic source. As age progresses, the MSC count in autologous sources decreases [57]. To achieve the desired cartilage regeneration by MSCs, allogeneic sources of MSCs can be isolated and culture-expanded to provide more MSCs and to provide off-the-shelf products to allow for emergency application. To have the desired cartilage regeneration, the optimal dose of cells, adjuvants, and source of MSC harvest has not been standardized.
Cell-based regenerative therapies rely on consistent, potent, and effective sources of MSCs. Various researchers have conducted clinical trials on embryonic stem cells and induced pluripotent stem cells (iPSCs). The parameters affecting the commercialization of iPSCs are cost, long-term culture and storage, and tumorigenic potential [58]. Autologous cell sources avoid host immune rejection for cell engraftment and retention. Autologous cell therapy requires multi-stage procedures for cell isolation, expansion, and transplanting back to target sites. The variability in the clinical outcome by using the autologous cell source depends on the subjective patient difference which is a major obstacle for reliability and quality control of the product [49]. To overcome all these obstacles, an allogeneic source of cells can be attempted as promising next-generation cell therapy.
Before selecting any allogeneic MSC product for cartilage regeneration, certain parameters should be considered such as MSC passage number, the desired number of MSCs to be injected, the immunogenicity of allogeneic MSCs, the shelf life of allogeneic MSC product, and injection and rehabilitation protocols [56]. Allogeneic preparation of MSCs has to be carried out in a Good Manufacturing Practice (GMP)-certified laboratory with the due regulatory guidelines and protocols [56]. The double negative cell population (HLA-1 negative and HLA-2 negative) renders an allogeneic pool of MSCs as the preferential therapeutic product in the field of regenerative medicine [59]. The temporal relationship between the efficacy of the MSCs delivered in cartilage regeneration with the number of cellular passages, dosage and frequency, cell of origin, usage of scaffolds, and bio-micromolecules has yet to be established. Having discussed the yield, safety, and efficacy of both the autologous and allogenic sources of MSCs, the final choice is always taken upon discussion with the patient based on the affordability and personal preferences.
2.2. Choice of Anaesthesia
The available literature lacks the standard guidelines or consensus for reducing the pain during bone marrow aspiration (BMA) and the post-procedural period. The pain experienced by more than 50% of the individuals who are undergoing BMA was neglected [60]. There are both dependent and independent factors in experiencing pain during bone marrow aspiration namely age, gender, body mass index (BMI), information regarding the procedure, previous BMA, site of BMA, the experience of the physician or surgeon, duration of BMA, and the level of difficulty in performing BMA [61]. A temporal association has been documented between duration and the level of difficulty in performing BMA [62]. The patients undergoing repeated BMA procedures were reported to endure unbearable pain [63]. An adequate level of anesthesia and analgesia is essential for any orthopedic surgery to be successful. For successful harvesting of bone marrow, monitored anesthesia (conscious sedation), regional anesthesia, and general anesthesia are the most widely used anesthesia techniques [61][64][65].
Various studies have used different local anesthetic agents [lidocaine, chloroprocaine, bupivacaine, articaine, or mepivacaine) to aspirate BM but reported no significant difference in the reduction of pain [66][67]. The coupling of intravenous sedation (IVS, lorazepam, midazolam, or diazepam) with local anesthesia reduces anxiety and pain perception [68][69] and IVS drugs also cause retrograde amnesia in some patients [70]. Holdsworth et al. documented less BMA pain and distress in patients receiving propofol/fentanyl general anesthesia than the eutectic mixture of local anesthetics (EMLA) or oral midazolam/EMLA [71]. Pretreatment with oral tramadol 50 mg 1 hour before BMA reduces procedural pain significantly while compared with oral placebo [62]. General anesthesia by propofol and fentanyl offers a good choice for short-term painful procedures in children undergoing treatment for BMA as a daycare procedure [65]. Deep sedation with midazolam, fentanyl, and propofol has proven benefits in the form of pain reduction [72]. The literature lacks the association between the choice of anesthesia and the yield of BMAC. Further studies are warranted to validate the yield of BMAC with the different choices of anesthesia used for the BMA procedure.
2.3. Site of Aspiration
Bone marrow and adipose tissue remain the commonly investigated source of MSCs for cartilage regeneration. The number of MSCs present in bone marrow is less when compared with adipose tissue [73]. The anterior and posterior iliac crests [8][74][75], the ilium [76][77], the proximal humerus [77][78], the proximal tibia [64][79], the distal femur [80][81], the distal tibia [82], the sternum [83][84], the mandible [85][86], and the calcaneum [87][88] are the most common locations for BMAC harvest without significant morbidity to the donor site. The primary site recommended to harvest BMAC is the iliac crest. Hernigou et al., defined the “zone model” and “sector rule” in pelvic bone for choosing the entry point to draw bone marrow [89]. This zone model divides the iliac crest into six different zones as sectors. With the help of CT scans, the sectors were defined based on the bone thickness, the maximum available bone depth for trocar purchase in these different parts of the iliac crest, and the corresponding vascular structures at risk. Hernigou et al., determined that the safe entry point should be approximately 2.5 cm distally from the anterior superior iliac spine (ASIS) [89]. Various studies have stated that BMAC is harvested from ASIS or the iliac crest in the supine position and posterior superior iliac crest (PSIS) in the prone position, respectively [8]. In children, the sternum, anterior iliac crest, tibia, and spinous process of the vertebra are the most common sites for bone marrow aspiration [75]. Bierman et al., reported that the posterior iliac crest is the safest site for harvesting bone marrow as it is the thickest portion of the posterior segment of the iliac crest with a huge amount of cancellous bony tissues [75]. The volume of bone marrow in the posterior iliac crest is more than the anterior iliac crest [75].
There is a longstanding ongoing debate on the harvesting technique of BMAC. Oliver et al. [90] stated that more BMAC cellular concentrations can be obtained by single insertion technique than multiple insertion technique whereas Peters et al. [91] commented that multiple insertions (up to four) resulted in a higher volume and concentration of BMAC cellular components. Kasashima et al. stated that the insertion of 5 mm of bone marrow needle into the equine sternum three times yielded more BM-MSCs with reduced peripheral blood contamination [92]. They further claimed that under USG guidance, the accurate placement of bone marrow needles into the medullary cavity facilitates the harvest of bone marrow with the least possible damage to the sternum [92].
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering9020057
References
- Kim, G.B.; Seo, M.-S.; Park, W.T.; Lee, G.W. Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3224.
- Martin, D.R.; Cox, N.R.; Hathcock, T.L.; Niemeyer, G.P.; Baker, H.J. Isolation and Characterization of Multipotential Mesenchymal Stem Cells from Feline Bone Marrow. Exp. Hematol. 2002, 30, 879–886.
- Chahla, J.; Dean, C.S.; Moatshe, G.; Pascual-Garrido, C.; Serra Cruz, R.; LaPrade, R.F. Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee: A Systematic Review of Outcomes. Orthop. J. Sports Med. 2016, 4, 2325967115625481.
- Muschler, G.F.; Nitto, H.; Boehm, C.A.; Easley, K.A. Age- and Gender-Related Changes in the Cellularity of Human Bone Marrow and the Prevalence of Osteoblastic Progenitors. J. Orthop. Res. 2001, 19, 117–125.
- Muschler, G.F.; Boehm, C.; Easley, K. Aspiration to Obtain Osteoblast Progenitor Cells from Human Bone Marrow: The Influence of Aspiration Volume. J. Bone Joint Surg Am. 1997, 79, 1699–1709.
- Piuzzi, N.S.; Hussain, Z.B.; Chahla, J.; Cinque, M.E.; Moatshe, G.; Mantripragada, V.P.; Muschler, G.F.; LaPrade, R.F. Variability in the Preparation, Reporting, and Use of Bone Marrow Aspirate Concentrate in Musculoskeletal Disorders: A Systematic Review of the Clinical Orthopaedic Literature. J. Bone Joint Surg Am. 2018, 100, 517–525.
- Hegde, V.; Shonuga, O.; Ellis, S.; Fragomen, A.; Kennedy, J.; Kudryashov, V.; Lane, J.M. A Prospective Comparison of 3 Approved Systems for Autologous Bone Marrow Concentration Demonstrated Nonequivalency in Progenitor Cell Number and Concentration. J. Orthop. Trauma 2014, 28, 591–598.
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc. Tech. 2017, 6, e441–e445.
- Gs, T.; Gd, C.; Im, K.; Ia, G.; Ms, T.; Pj, P.; Od, S. Effectiveness of a Single Intra-Articular Bone Marrow Aspirate Concentrate (BMAC) Injection in Patients with Grade 3 and 4 Knee Osteoarthritis. Heliyon 2018, 4.
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of Knee Osteoarthritis with Autologous Mesenchymal Stem Cells: Two-Year Follow-up Results. Transplantation 2014, 97, e66–e68.
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of Knee Osteoarthritis With Autologous Mesenchymal Stem Cells: A Pilot Study. Transplantation 2013, 95, 1535–1541.
- Hussein, M.; van Eck, C.F.; Kregar Velikonja, N. Bone Marrow Aspirate Concentrate Is More Effective Than Hyaluronic Acid and Autologous Conditioned Serum in the Treatment of Knee Osteoarthritis: A Retrospective Study of 505 Consecutive Patients. Appl. Sci. 2021, 11, 2932.
- Cavallo, C.; Boffa, A.; Andriolo, L.; Silva, S.; Grigolo, B.; Zaffagnini, S.; Filardo, G. Bone Marrow Concentrate Injections for the Treatment of Osteoarthritis: Evidence from Preclinical Findings to the Clinical Application. Int. Orthop. 2021, 45, 525–538.
- Lavender, C.; Johnson, B.; Kopiec, A. Augmentation of Anterior Cruciate Ligament Reconstruction With Bone Marrow Concentrate and a Suture Tape. Arthrosc. Tech. 2018, 7, e1289–e1293.
- Koch, M.; Hammer, S.; Fuellerer, J.; Lang, S.; Pfeifer, C.G.; Pattappa, G.; Weber, J.; Loibl, M.; Nerlich, M.; Angele, P.; et al. Bone Marrow Aspirate Concentrate for the Treatment of Avascular Meniscus Tears in a One-Step Procedure—Evaluation of an In Vivo Model. Int. J. Mol. Sci. 2019, 20, 1120.
- Imam, M.A.; Holton, J.; Horriat, S.; Negida, A.S.; Grubhofer, F.; Gupta, R.; Narvani, A.; Snow, M. A Systematic Review of the Concept and Clinical Applications of Bone Marrow Aspirate Concentrate in Tendon Pathology. SICOT J. 2017, 3, 58.
- Palombella, S.; Lopa, S.; Gianola, S.; Zagra, L.; Moretti, M.; Lovati, A.B. Bone Marrow-Derived Cell Therapies to Heal Long-Bone Nonunions: A Systematic Review and Meta-Analysis—Which Is the Best Available Treatment? Stem Cells Int. 2019, 2019, e3715964.
- Gianakos, A.; Ni, A.; Zambrana, L.; Kennedy, J.G.; Lane, J.M. Bone Marrow Aspirate Concentrate in Animal Long Bone Healing: An Analysis of Basic Science Evidence. J. Orthop. Trauma 2016, 30, 1–9.
- Neubauer, M.; Jeyakumar, V.; Muellner, T.; Nehrer, S. Bone-Marrow-Aspirate-Concentrate for Chondral Defects: Surgical Techniques, Clinical Applications and Basic Science. Ann. Joint 2018, 3, 107.
- Subaşı, V.; Ekiz, T. Bone Marrow Aspiration Concentrate and Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Report of Three Cases. Complement Ther. Clin. Pract 2019, 34, 113–115.
- Centeno, C.; Pitts, J.; Al-Sayegh, H.; Freeman, M. Efficacy of Autologous Bone Marrow Concentrate for Knee Osteoarthritis with and without Adipose Graft. BioMed Res. Int. 2014, 2014, 370621.
- Jeyaraman, M.; Muthu, S.; Jain, R.; Khanna, M. Autologous Bone Marrow Derived Mesenchymal Stem Cell Therapy for Osteonecrosis of Femoral Head: A Systematic Overview of Overlapping Meta-Analyses. J. Clin. Orthop. Trauma 2021, 13, 134–142.
- Betzler, B.K.; Bin Muhammad Ridzwan Chew, A.H.; Bin Abd Razak, H.R. Intra-Articular Injection of Orthobiologics in Patients Undergoing High Tibial Osteotomy for Knee Osteoarthritis Is Safe and Effective—A Systematic Review. J. Exp. Orthop. 2021, 8, 83.
- Goyal, D. Recent Advances and Future Trends in Articular Cartilage Repair. JASSM 2020, 1, 159–173.
- Kennedy, J.G.; Murawski, C.D. The Treatment of Osteochondral Lesions of the Talus with Autologous Osteochondral Transplantation and Bone Marrow Aspirate Concentrate: Surgical Technique. Cartilage 2011, 2, 327–336.
- Eder, C.; Schmidt-Bleek, K.; Geissler, S.; Sass, F.A.; Maleitzke, T.; Pumberger, M.; Perka, C.; Duda, G.N.; Winkler, T. Mesenchymal Stromal Cell and Bone Marrow Concentrate Therapies for Musculoskeletal Indications: A Concise Review of Current Literature. Mol. Biol. Rep. 2020, 47, 4789–4814.
- Dragoo, J.L.; Guzman, R.A. Evaluation of the Consistency and Composition of Commercially Available Bone Marrow Aspirate Concentrate Systems. Orthop. J. Sports Med. 2020, 8, 2325967119893634.
- Schäfer, R.; DeBaun, M.R.; Fleck, E.; Centeno, C.J.; Kraft, D.; Leibacher, J.; Bieback, K.; Seifried, E.; Dragoo, J.L. Quantitation of Progenitor Cell Populations and Growth Factors after Bone Marrow Aspirate Concentration. J. Transl. Med. 2019, 17, 115.
- Hernigou, J.; Vertongen, P.; Rasschaert, J.; Hernigou, P. Role of Scaffolds, Subchondral, Intra-Articular Injections of Fresh Autologous Bone Marrow Concentrate Regenerative Cells in Treating Human Knee Cartilage Lesions: Different Approaches and Different Results. Int. J. Mol. Sci. 2021, 22, 3844.
- Kon, E.; Boffa, A.; Andriolo, L.; Di Martino, A.; Di Matteo, B.; Magarelli, N.; Marcacci, M.; Onorato, F.; Trenti, N.; Zaffagnini, S.; et al. Subchondral and Intra-Articular Injections of Bone Marrow Concentrate Are a Safe and Effective Treatment for Knee Osteoarthritis: A Prospective, Multi-Center Pilot Study. Knee Surg Sports Traumatol. Arthrosc. 2021, 29, 4232–4240.
- Chen, M.J.; Whiteley, J.P.; Please, C.P.; Ehlicke, F.; Waters, S.L.; Byrne, H.M. Identifying Chondrogenesis Strategies for Tissue Engineering of Articular Cartilage. J. Tissue Eng. 2019, 10, 2041731419842431.
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Tian, L.; Shamirzaei-Jeshvaghani, E.; Dehghani, L.; Ramakrishna, S. Structural Properties of Scaffolds: Crucial Parameters towards Stem Cells Differentiation. World J. Stem. Cells 2015, 7, 728–744.
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A Review. Bioact. Mater. 2019, 4, 271–292.
- Alaribe, F.N.; Manoto, S.L.; Motaung, S.C.K.M. Scaffolds from Biomaterials: Advantages and Limitations in Bone and Tissue Engineering. Biologia 2016, 71, 353–366.
- Rikkers, M.; Levato, R.; Malda, J.; Vonk, L.A. Importance of Timing of Platelet Lysate-Supplementation in Expanding or Redifferentiating Human Chondrocytes for Chondrogenesis. Front. Bioeng. Biotechnol. 2020, 8, 804.
- Naskou, M.C.; Sumner, S.M.; Chocallo, A.; Kemelmakher, H.; Thoresen, M.; Copland, I.; Galipeau, J.; Peroni, J.F. Platelet Lysate as a Novel Serum-Free Media Supplement for the Culture of Equine Bone Marrow-Derived Mesenchymal Stem Cells. Stem. Cell Res. Ther. 2018, 9, 75.
- Lippross, S.; Loibl, M.; Hoppe, S.; Meury, T.; Benneker, L.; Alini, M.; Verrier, S. Platelet Released Growth Factors Boost Expansion of Bone Marrow Derived CD34(+) and CD133(+) Endothelial Progenitor Cells for Autologous Grafting. Platelets 2011, 22, 422–432.
- Philippart, P.; Meuleman, N.; Stamatopoulos, B.; Najar, M.; Pieters, K.; De Bruyn, C.; Bron, D.; Lagneaux, L. In Vivo Production of Mesenchymal Stromal Cells After Injection of Autologous Platelet-Rich Plasma Activated by Recombinant Human Soluble Tissue Factor in the Bone Marrow of Healthy Volunteers. Tissue Eng. Part A 2014, 20, 160–170.
- Griffiths, S.; Baraniak, P.R.; Copland, I.B.; Nerem, R.M.; McDevitt, T.C. Human Platelet Lysate Stimulates High-Passage and Senescent Human Multipotent Mesenchymal Stromal Cell Growth and Rejuvenation in Vitro. Cytotherapy 2013, 15, 1469–1483.
- Seo, J.; Tsuzuki, N.; Haneda, S.; Yamada, K.; Furuoka, H.; Tabata, Y.; Sasaki, N. Comparison of Allogeneic Platelet Lysate and Fetal Bovine Serum for in Vitro Expansion of Equine Bone Marrow-Derived Mesenchymal Stem Cells. Res. Vet. Sci. 2013, 95, 693–698.
- Kim, G.B.; Kim, J.-D.; Choi, Y.; Choi, C.H.; Lee, G.W. Intra-Articular Bone Marrow Aspirate Concentrate Injection in Patients with Knee Osteoarthritis. Appl. Sci. 2020, 10, 5945.
- Kim, J.-D.; Lee, G.W.; Jung, G.H.; Kim, C.K.; Kim, T.; Park, J.H.; Cha, S.S.; You, Y.-B. Clinical Outcome of Autologous Bone Marrow Aspirates Concentrate (BMAC) Injection in Degenerative Arthritis of the Knee. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 1505–1511.
- Gobbi, A.; Karnatzikos, G.; Scotti, C.; Mahajan, V.; Mazzucco, L.; Grigolo, B. One-Step Cartilage Repair with Bone Marrow Aspirate Concentrated Cells and Collagen Matrix in Full-Thickness Knee Cartilage Lesions. Cartilage 2011, 2, 286–299.
- Enea, D.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Manzotti, S.; Kaps, C.; Gigante, A. Single-Stage Cartilage Repair in the Knee with Microfracture Covered with a Resorbable Polymer-Based Matrix and Autologous Bone Marrow Concentrate. Knee 2013, 20, 562–569.
- Krych, A.J.; Nawabi, D.H.; Farshad-Amacker, N.A.; Jones, K.J.; Maak, T.G.; Potter, H.G.; Williams, R.J. Bone Marrow Concentrate Improves Early Cartilage Phase Maturation of a Scaffold Plug in the Knee: A Comparative Magnetic Resonance Imaging Analysis to Platelet-Rich Plasma and Control. Am. J. Sports Med. 2016, 44, 91–98.
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90.
- Guilak, F. Biomechanical Factors in Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823.
- Jackson, B.D.; Wluka, A.E.; Teichtahl, A.J.; Morris, M.E.; Cicuttini, F.M. Reviewing Knee Osteoarthritis--a Biomechanical Perspective. J. Sci. Med. Sport 2004, 7, 347–357.
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 68.
- García-Bernal, D.; García-Arranz, M.; Yáñez, R.M.; Hervás-Salcedo, R.; Cortés, A.; Fernández-García, M.; Hernando-Rodríguez, M.; Quintana-Bustamante, Ó.; Bueren, J.A.; García-Olmo, D.; et al. The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy. Front. Cell Dev. Biol. 2021, 9, 650664.
- Park, Y.; Ha, C.; Lee, C.; Yoon, Y.C.; Park, Y. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621.
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and Safety of Adult Human Bone Marrow-Derived, Cultured, Pooled, Allogeneic Mesenchymal Stromal Cells (Stempeucel®): Preclinical and Clinical Trial in Osteoarthritis of the Knee Joint. Arthritis Res. Ther. 2016, 18, 301.
- Weiss, J.N. A Phase 3 Study to Evaluate the Efficacy and Safety of JointStem in the Treatment of Osteoarthritis. In Orthopedic Stem Cell Surgery; Weiss, J.N., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 199–203. ISBN 978-3-030-73299-8.
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, e9628536.
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Trans. 2019, 28, 801–812.
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The Challenges and Promises of Allogeneic Mesenchymal Stem Cells for Use as a Cell-Based Therapy. Stem Cell Res. Ther. 2015, 6, 234.
- Yu, J.M.; Wu, X.; Gimble, J.M.; Guan, X.; Freitas, M.A.; Bunnell, B.A. Age-Related Changes in Mesenchymal Stem Cells Derived from Rhesus Macaque Bone Marrow. Aging Cell 2011, 10, 66–79.
- Moradi, S.; Mahdizadeh, H.; Šarić, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B. Research and Therapy with Induced Pluripotent Stem Cells (IPSCs): Social, Legal, and Ethical Considerations. Stem Cell Res. Ther. 2019, 10, 341.
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal Stem Cells Avoid Allogeneic Rejection. J. Inflamm. 2005, 2, 8.
- Kuball, J.; Schüz, J.; Gamm, H.; Weber, M. Bone Marrow Punctures and Pain. Acute Pain 2004, 6, 9–14.
- Hjortholm, N.; Jaddini, E.; Hałaburda, K.; Snarski, E. Strategies of Pain Reduction during the Bone Marrow Biopsy. Ann. Hematol. 2013, 92, 145–149.
- Vanhelleputte, P.; Nijs, K.; Delforge, M.; Evers, G.; Vanderschueren, S. Pain during Bone Marrow Aspiration: Prevalence and Prevention. J. Pain Symptom Manag. 2003, 26, 860–866.
- Degen, C.; Christen, S.; Rovo, A.; Gratwohl, A. Bone Marrow Examination: A Prospective Survey on Factors Associated with Pain. Ann. Hematol. 2010, 89, 619–624.
- Abla, O.; Friedman, J.; Doyle, J. Performing Bone Marrow Aspiration and Biopsy in Children: Recommended Guidelines. Paediatr Child Health 2008, 13, 499–501.
- Ghasemi, A.; Gharavi Fard, M.; Sabzevari, A. General Anesthesia for Lumbar Puncture and Bone Marrow Aspiration /Biopsy in Children with Cancer. Iran J. Ped Hematol. Oncol. 2013, 3, 54–58.
- Riley, R.S.; Hogan, T.F.; Pavot, D.R.; Forysthe, R.; Massey, D.; Smith, E.; Wright, L.; Ben-Ezra, J.M. A Pathologist’s Perspective on Bone Marrow Aspiration and Biopsy: I. Performing a Bone Marrow Examination. J. Clin. Lab. Anal. 2004, 18, 70–90.
- Kuivalainen, A.-M.; Niemi-Murola, L.; Widenius, T.; Elonen, E.; Rosenberg, P.H. Comparison of Articaine and Lidocaine for Infiltration Anaesthesia in Patients Undergoing Bone Marrow Aspiration and Biopsy. Eur. J. Pain 2010, 14, 160–163.
- Friedman, A.G.; Mulhern, R.K.; Fairclough, D.; Ward, P.M.; Baker, D.; Mirro, J.; Rivera, G.K. Midazolam Premedication for Pediatric Bone Marrow Aspiration and Lumbar Puncture. Med. Pediatr. Oncol. 1991, 19, 499–504.
- Chakupurakal, G.; Delgado, J.; Nikolousis, E.; Pitchapillai, S.; Allotey, D.; Holder, K.; Bratby, L.; de la Rue, J.; Milligan, D.W. Midazolam in Conjunction with Local Anaesthesia Is Superior to Entonox in Providing Pain Relief during Bone Marrow Aspirate and Trephine Biopsy. J. Clin. Pathol. 2008, 61, 1051–1054.
- Milligan, D.W.; Howard, M.R.; Judd, A. Premedication with Lorazepam before Bone Marrow Biopsy. J. Clin. Pathol. 1987, 40, 696–698.
- Holdsworth, M.T.; Raisch, D.W.; Winter, S.S.; Frost, J.D.; Moro, M.A.; Doran, N.H.; Phillips, J.; Pankey, J.M.; Mathew, P. Pain and Distress from Bone Marrow Aspirations and Lumbar Punctures. Ann. Pharmacother. 2003, 37, 17–22.
- Burkle, C.M.; Harrison, B.A.; Koenig, L.F.; Decker, P.A.; Warner, D.O.; Gastineau, D.A. Morbidity and Mortality of Deep Sedation in Outpatient Bone Marrow Biopsy. Am. J. Hematol. 2004, 77, 250–256.
- Alonso-Goulart, V.; Ferreira, L.B.; Duarte, C.A.; de Lima, I.L.; Ferreira, E.R.; de Oliveira, B.C.; Vargas, L.N.; de Moraes, D.D.; Silva, I.B.B.; de Oliveira Faria, R.; et al. Mesenchymal Stem Cells from Human Adipose Tissue and Bone Repair: A Literature Review. Biotechnol. Res. Innovation 2018, 2, 74–80.
- Dimitriou, R.I.; Kanakaris, N.K.; Giannoudis, P.V. Percutaneous Bone Marrow Aspirate Harvesting from the Anterior Iliac Crest. In Practical Procedures in Orthopedic Surgery: Joint Aspiration/Injection, Bone Graft Harvesting and Lower Limb Amputations; Giannoudis, P.V., Ed.; Springer: London, UK, 2012; pp. 45–49. ISBN 978-0-85729-817-1.
- Bierman, H.R. BONE MARROW ASPIRATION—The Posterior Iliac Crest, an Additional Safe Site. Calif Med. 1952, 77, 138–139.
- Hernigou, J.; Alves, A.; Homma, Y.; Guissou, I.; Hernigou, P. Anatomy of the Ilium for Bone Marrow Aspiration: Map of Sectors and Implication for Safe Trocar Placement. Int. Orthop. 2014, 38, 2585–2590.
- Otto, A.; Muench, L.N.; Kia, C.; Baldino, J.B.; Mehl, J.; Dyrna, F.; Voss, A.; McCarthy, M.B.; Nazal, M.R.; Martin, S.D.; et al. Proximal Humerus and Ilium Are Reliable Sources of Bone Marrow Aspirates for Biologic Augmentation During Arthroscopic Surgery. Arthrosc.: J. Arthrosc. Related Surg. 2020, 36, 2403–2411.
- Voss, A.; McCarthy, M.B.; Singh, H.; Beitzel, K.; DiVenere, J.; Cote, M.P.; Hoberman, A.R.; Nowak, M.; Imhoff, A.B.; Mazzocca, A.D. The Influence of Trocar Fenestration and Volume on Connective Tissue Progenitor Cells (Stem Cells) in Arthroscopic Bone Marrow Aspiration From the Proximal Humerus. Arthroscopy 2017, 33, 1167–1174.e1.
- Narbona-Carceles, J.; Vaquero, J.; Suárez-Sancho, S.B.S.; Forriol, F.; Fernández-Santos, M.E. Bone Marrow Mesenchymal Stem Cell Aspirates from Alternative Sources: Is the Knee as Good as the Iliac Crest? Injury 2014, 45 Suppl 4, S42–S47.
- Beitzel, K.; McCarthy, M.B.R.; Cote, M.P.; Durant, T.J.S.; Chowaniec, D.M.; Solovyova, O.; Russell, R.P.; Arciero, R.A.; Mazzocca, A.D. Comparison of Mesenchymal Stem Cells (Osteoprogenitors) Harvested from Proximal Humerus and Distal Femur during Arthroscopic Surgery. Arthroscopy 2013, 29, 301–308.
- Juneja, S.C.; Viswanathan, S.; Ganguly, M.; Veillette, C. A Simplified Method for the Aspiration of Bone Marrow from Patients Undergoing Hip and Knee Joint Replacement for Isolating Mesenchymal Stem Cells and In Vitro Chondrogenesis. Bone Marrow Res. 2016, 2016, e3152065.
- Daigre, J.L.; DeMill, S.L.; Hyer, C.F. Assessment of Bone Marrow Aspiration Site Pain in Foot and Ankle Surgery. Foot Ankle Spec. 2016, 9, 215–217.
- Trejo-Ayala, R.A.; Luna-Pérez, M.; Gutiérrez-Romero, M.; Collazo-Jaloma, J.; Cedillo-Pérez, M.C.; Ramos-Peñafiel, C.O. Bone Marrow Aspiration and Biopsy. Technique and Considerations. Revista Médica Del Hospital General De México 2015, 78, 196–201.
- Asakura, Y.; Kinoshita, M.; Kasuya, Y.; Sakuma, S.; Ozaki, M. Ultrasound-Guided Sternal Bone Marrow Aspiration. Blood Res. 2017, 52, 148–150.
- Lee, B.-K.; Choi, S.-J.; Mack, D.; Oh, S.-H. Isolation of Mesenchymal Stem Cells from the Mandibular Marrow Aspirates. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, e86–e93.
- Hong, Y.; Xu, H.; Yang, Y.; Zhou, S.; Jin, A.; Huang, X.; Dai, Q.; Jiang, L. Isolation and Cultivation of Mandibular Bone Marrow Mesenchymal Stem Cells in Rats. J. Vis. Exp. 2020.
- Li, C.; Kilpatrick, C.D.; Smith, S.; Glettig, D.L.; Glod, D.J.; Mallette, J.; Strunk, M.R.; Chang, J.; Angle, S.R.; Kaplan, D.L. Assessment of Mesenchymal Stem Cells in Bone Marrow Aspirate from Human Calcaneus. J. Foot Ankle Surg. 2017, 56, 42–46.
- Hyer, C.F.; Berlet, G.C.; Bussewitz, B.W.; Hankins, T.; Ziegler, H.L.; Philbin, T.M. Quantitative Assessment of the Yield of Osteoblastic Connective Tissue Progenitors in Bone Marrow Aspirate from the Iliac Crest, Tibia, and Calcaneus. J. Bone Joint Surg. Am. 2013, 95, 1312–1316.
- Hernigou, J.; Picard, L.; Alves, A.; Silvera, J.; Homma, Y.; Hernigou, P. Understanding Bone Safety Zones during Bone Marrow Aspiration from the Iliac Crest: The Sector Rule. Int. Orthop. 2014, 38, 2377–2384.
- Oliver, K.; Awan, T.; Bayes, M. Single- Versus Multiple-Site Harvesting Techniques for Bone Marrow Concentrate: Evaluation of Aspirate Quality and Pain. Orthop. J. Sports Med. 2017, 5, 2325967117724398.
- Peters, A.E.; Watts, A.E. Biopsy Needle Advancement during Bone Marrow Aspiration Increases Mesenchymal Stem Cell Concentration. Front. Vet. Sci. 2016, 3, 23.
- Kasashima, Y.; Ueno, T.; Tomita, A.; Goodship, A.E.; Smith, R.K.W. Optimisation of Bone Marrow Aspiration from the Equine Sternum for the Safe Recovery of Mesenchymal Stem Cells. Equine Vet. J. 2011, 43, 288–294.
This entry is offline, you can click here to edit this entry!
