If a bacterium has motility, it will use the ability to survive and thrive. For many pathogenic species, their motilities are a crucial virulence factor. The form of motility varies among the species. Some use flagella for swimming in liquid, and others use the cell-surface machinery to move over solid surfaces. Spirochetes are distinguished from other bacterial species by their helical or flat wave morphology and periplasmic flagella (PFs). It is believed that the rotation of PFs beneath the outer membrane causes transformation or rolling of the cell body, propelling the spirochetes. Interestingly, some spirochetal species exhibit motility both in liquid and over surfaces, but it is not fully unveiled how the spirochete pathogenicity involves such amphibious motility.
1. Introduction
Many species of bacteria have motility operated with diverse mechanisms [
1,
2]. The flagellum is one of the major motility organs that are used by
Escherichia coli,
Salmonella spp.,
Helicobacter pylori,
Pseudomonas aeruginosa, and others [
3].
Mycoplasma spp. and
Myxococcus xanthus move on surfaces, called gliding motility, and this is achieved via the molecular architecture on the cell surface [
4,
5].
P. aeruginosa and unicellular cyanobacteria also show motility on surfaces using extension and contraction of pili [
6,
7]. These bacteria rely on motility for navigation to explore preferred environments for growing, and pathogenic species utilize this ability for invading hosts [
8,
9].
Spirochetes are a group of Gram-negative bacteria and include pathogenic species, such as Treponema pallidum (syphilis), Treponema denticola (periodontal disease), Brachyspira hyodysenteriae (swine dysentery), Borrelia burgdorferi (Lyme disease), and Leptospira interrogans (leptospirosis). The spirochetes exhibit helical (e.g., Leptospira spp.) or flat-wave (e.g., Borrelia spp.) cell morphology and have multiple flagella within the periplasmic space. The spirochete flagella are called periplasmic flagella (PFs). The PF-dependent motility is known to be an essential virulence factor, but the mechanism of how spirochetes use the motility in the pathogenic process has not been fully understood.
2. Leptospirosis
Pathogenic leptospires colonize the proximal renal tubules of animals recovering from the disease, or reservoir hosts such as rodents. The bacteria are shed upon urination into environments, infecting animals in contact with the contaminated soil or water through injured skin. Diverse species of mammals have the potential to acquire
Leptospira infection. Pathogenic
Leptospira are classified into more than 300 serovars based on the structure of lipopolysaccharide (LPS), and the severity of the resultant symptom depends on the combination of
Leptospira serovars and host species. In severe cases, the penetrating leptospires reach specific organs, such as the lung, liver, and kidney, causing hemorrhage, jaundice, and nephritis [
10,
11,
12]. Animal experiments have shown that the loss-of-motility due to the knock-out of the PF-related genes reduces the virulence of
Leptospira, suggesting that motility is an essential factor determining the pathogenicity [
13,
14].
3. Morphology and Motility of Leptospira
3.1. Cell Morphology
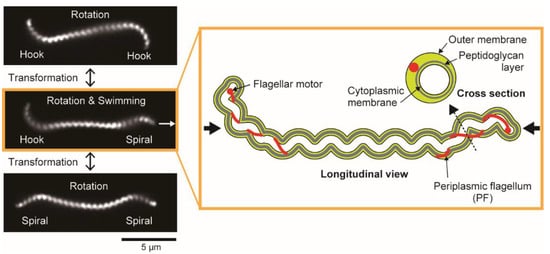
Leptospira spp. have two PFs (1 PF/cell end) within a thin (~150 nm in diameter), long (~20 μm in length), and short-pitch helical cell body (~700 nm in wavelength). The extracted PFs from the cell body exhibit a coiled shape, thus giving the cell ends curvature. The cell-end morphology depends on the gyration direction of the cell ends: Gyrating counterclockwise (CCW, viewing the cellular tip as indicated by thick black arrows in the cartoon of Figure 1) and clockwise (CW), the cell end form a “hook” shape and “spiral” shape, respectively (Figure 1) [15].
Figure 1. Morphology and motion forms of Leptospira interrogans. Dark-field micrographs (left) show three motion modes observed in the same cell with distinct cell-end morphologies. The cell rotates without net migration when exhibiting symmetric forms (Spiral–Spiral or Hook–Hook). Asymmetric form (Spiral–Hook) propels the cell in the direction indicated by the white arrow. Longitudinal and cross-sectional views of the asymmetric swimming mode are schematically depicted on the right. Thick black arrows indicate that the directions of cell-end gyration are defined by viewing the cellular tip from the cell exterior (see main text).
3.2. Swimming
Asymmetric configuration of the cell body propels the cell unidirectionally, and the anterior and posterior cell-body ends exhibit the spiral and hook shapes, respectively. The cell-end morphology frequently switches with the reversal of gyration, allowing the cell to change swimming direction.
Leptospira often shows symmetric morphology (i.e., Spiral–Spiral or Hook–Hook), then rotating without net migration (
Figure 1) [
15,
16]. Though the PF rotation has not been observed directly, its counter-torque is thought to turn the entire protoplasmic cylinder (PC). The combination of PC rotation (
~
100 Hz) and the spiral-end gyration (
~50 Hz) produces thrust for swimming [
17]. Motility assays showed that the migration distance by one revolution of PC is
~
30% of the wavelength of the PC in a water-based solution, indicating that the swimming of
Leptospira is a slippery motion [
18]. However, in gel-like viscoelastic fluids (e.g., methylcellulose solution), the motion efficiency of
Leptospira is improved up to 100% [
15], resulting in an increment of the swimming velocity [
19]. Interestingly, the addition of viscous agent to medium increases the frequency of swimming reversal, as discussed later [
16,
20].
3.3. Crawling
In 1975, Cox and Twigg showed a trail of the leptospiral movement on a smooth surface and called it “crawling” [
21]. Charon et al. reported that unidentified outer membrane components have mobility along the cell body by observing the movement of microbeads attached to the cell surface via an anti-whole cell antibody [
22]. Recently, Tahara et al. revealed that crawling is: (i) PF-dependent motility; (ii) conducted by only PC rotation without the direct contribution of the spiral end; (iii) mediated by adhesive mobile components residing in the outer membrane, such as lipopolysaccharide; and (iv) utterly slip-less motion [
18]. Potential as a virulence factor of the
Leptospira crawling will be discussed below.
4. Swimming in a Highly Viscous Milieu
4.1. Dependence on a Type of Polymer
The effect of viscosity on bacterial swimming has been investigated in many species [
23], but we should note the type of polymers added to the media. For example, Ficoll, a highly branched polymer, makes a homogeneous viscous solution. In contrast, methylcellulose forms an elastic network in solution, and the bacterial movement in such a gel-like heterogeneous fluid depends on the size of the polymer network and bacteria [
24,
25]. Experiments and theoretical studies have shown that bacterial swimming in a gel-like fluid is accelerated monotonically or up to a certain point of the added polymer concentration, whereas the swimming speed decreases in Ficoll solutions [
17,
19,
23,
25,
26,
27,
28]. The heterogeneous, viscoelastic milieu is ubiquitous in the host body (e.g., mucus layers covering tissues and extracellular matrix), implying the significance of swimming in such unique environments for pathogenicity.
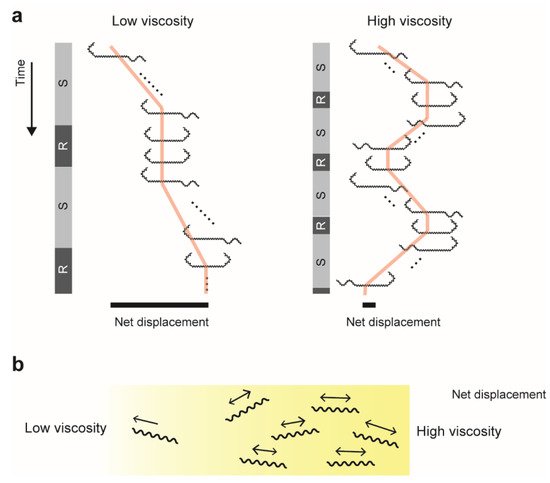
4.2. Back-and-Forth Motion
In polymer solutions,
Leptospira shows the speed variation in swimming [
17,
19,
29] and increases the transition frequency between swimming (Spiral–Hook) and rotation (Spiral–Spiral and Hook–Hook) modes [
16]. The enhancement of the motion-mode switching is observed in Ficoll, methylcellulose, and mucin solutions [
16]. In addition, we revealed that the swimming direction reverses more frequently in high viscosity (
Figure 2a) [
16,
20]. Enhancing “back and forth” movement suggests the limitation of the net migration, crowding bacteria within the mucus layer, and facilitating colonization over the tissues (
Figure 2b).
Figure 2. Enhancement of swimming reversal in high viscosity: (
a) Schematic explanations of transition between swimming (S) and rotation (R) modes. For simplicity, the rotation mode indicates only Hook–Hook morphology. Time courses of cell positions show the effect of reversal on net displacement (black bars). (
b) Accumulation of bacteria in high viscosity due to limitation of net migration. See [
16] for more details.
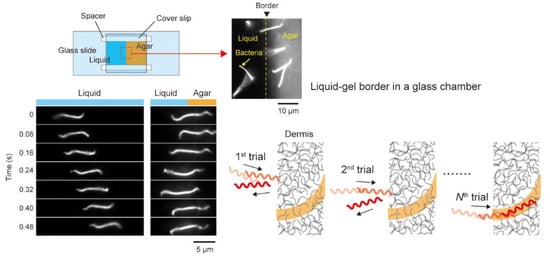
4.3. Trial and Error?
The previous section describes the back-and-forth motion in viscous media, but the behavior is also observed at the liquid and gel interface (
Figure 3) [
20]. The mechanism sensing viscosity remains unknown, but the fact indicates that penetration of not the entire but partial cell body to a gel-like milieu allows
Leptospira to change the swimming pattern. The experimental setup of the liquid–gel interface resembles wound skin exposed to environments contaminated by
Leptospira. A time record of the
Leptospira movement in the liquid–gel interface showed that the bacteria repeatedly attacked the interface while reversing the swimming direction and finally invading the gel phase (
Figure 3). Swimming reversal induced in the liquid–gel interface could be interpreted as “trial-and-error” to search for an easier route for invasion.
Figure 3. Swimming reversal enhanced in the liquid–gel border. The schematic (right bottom) explains a hypothetical scenario that the “trial-and-error”-like behavior allows the bacteria to find a path for easier invasion. This figure was made based on [
20] with modifications.
4.4. Interaction between PFs
Leptospira switches swimming direction within <1 s [
20]. Such a quick reversal suggests the coordination between two PFs mediated by unidentified signal transduction, but there is no definitive evidence so far. The most general signaling for the reversal of flagellar rotation involves the Che system: Sensing environmental stimuli via receptors induces phosphorylation (P) of the regulator protein CheY, and the binding of CheY-P to the flagellar motor reverses rotation. Noting that the diffusion constant (
D) of CheY-P is
~10 μm
2/s [
30], the theoretical estimation using the formula
t=
x2/2
D, where
t is the time for traveling the distance
x with
D, predicts the time gap of
~50 ms between the reversals of two flagellar motors in the same E. coli cell (
x~1 μm). In contrast, since the distance between PFs of
Leptospira is
~20 μm, the reversal upon CheY-P binding at one PF delays
~20 s from the other PF, indicating that the observed rapid reversal cannot be achieved only by the Che system. PFs of
Bo. burgdorferi is so long that they overlap at the center of the cell body [
31]. A theoretical study predicted that direct interaction between PFs is indispensable for propelling the
Bo. burgdorferi smoothly [
32]. In contrast, since PFs of
Leptospira are too short for contact with each other directly, the cell body might mediate the interaction between PFs [
17,
33]. There remains the possibility that
Leptospira switches the PF rotation and transforms the cell end (Spiral or Hook shape) at random, and the asymmetric swimming mode (Spiral–Hook) appears accidentally. However, the simple scenario will not explain the viscosity dependence of the swimming reversal [
16,
20]. Higher-resolution analysis focusing on the reversal timing of two PFs (cell-end gyration) may give a clue to unveil this long-term mystery.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031859