Carcinoid Crisis represents a rare and extremely dangerous manifestation that can occur in patients with Neuroendocrine Tumors (NETs). It is characterized by a sudden onset of hemodynamic instability, sometimes associated with the classical symptoms of carcinoid syndrome, such as bronchospasm and flushing. Carcinoid Crisis seems to be caused by a massive release of vasoactive substances, typically produced by neuroendocrine cells, and can emerge after abdominal procedures, but also spontaneously in rare instances. To date, there are no empirically derived guidelines for the management of this cancer-related medical emergency, and the available evidence essentially comes from single-case reports or dated small retrospective series. A transfer to the Intensive Care Unit may be necessary during the acute setting, when the severe hypotension becomes unresponsive to standard practices, such as volemic filling and the infusion of vasopressor therapy. The only effective strategy is represented by prevention. The administration of octreotide, anxiolytic and antihistaminic agents represents the current treatment approach to avoid hormone release and prevent major complications.
- crcinoid crisis
- neuroendocrine tumors
- hemodynamic instability
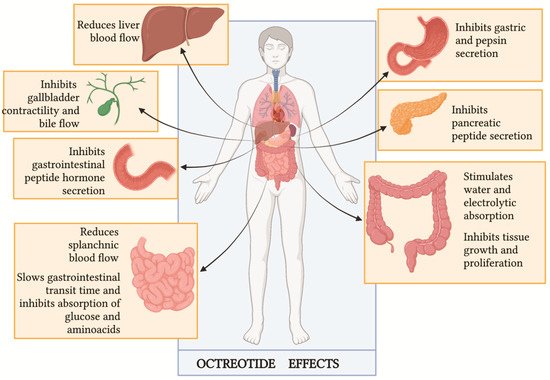
1. Octreotide
| Variation | Type of Paper | Number of Patients | Number of CC | Octreotide Dose and Regimen |
|---|---|---|---|---|
| Kvols et al., 1986 [3] | Case report-retrospective study | 25 | 1 | a bolus of 50 μg of octreotide intraoperatively |
| Kinney et al. [8] | Retrospective study | 119 | 15 (none of the pts received onctreotide intraoperatively) |
|
| Massimino et al. [9] | Retrospective study | 97 | 23 | 87 pts received prophylactic octreotide (median dose 500 μg—range 100–1100 μg) + intraoperative bolus if necessary (median dose 350 μg—range 100–5500 μg) |
| Woltering et al. [10] | Retrospective study | 150 | 6 | Continuous high-dose octreotide infusion: 500 μg/h |
| Condron et al. [11] | Prospective study | 127 | 38 | Continuous high-dose octreotide infusion: 100 μg/h |
| Kinney et al. [12] | Retrospective study | 169 | 0 |
|
| Kwon et al. [13] | Retrospective study | 75 | 24 |
|
- -
-
The evaluation of nutritional assessment with the diagnosis and correction of hydro electrolytic disorders, malnutrition and malabsorption, and the avoidance of food triggers and intensive physical exercises the previous day;
- -
-
The evaluation of NET characteristics (high tumor burden, use of somatostatin analogs to control CS).
2. Vasopressors
This entry is adapted from the peer-reviewed paper 10.3390/cancers14030662
References
- Borna, R.M.; Jahr, J.S.; Kmiecik, S.; Mancuso, K.F.; Kaye, A.D. Pharmacology of octreotide: Clinical implications for anesthe-siologists and associated risks. Anesthesiol. Clin. 2017, 35, 327–339.
- Lamberts, S.W.; van der Lely, A.J.; de Herder, W.W.; Hofland, L.J. Octreotide. N. Engl. J. Med. 1996, 334, 246–254.
- Kvols, L.K.; Martin, J.K.; Marsh, H.M.; Moertel, C.G. Rapid reversal of carcinoid crisis with somatostatin analogue. N. Engl. J. Med. 1985, 313, 1229.
- Kvols, L.K.; Moertel, C.G.; O’Connell, M.J.; Schutt, A.J.; Rubin, J.; Hahn, R.G. Treatment of the malignant carcinoid syndrome evaluation of a long-acting somatostatin analogue. N. Engl. J. Med. 1986, 315, 663–666.
- Warner, R.R.; Mani, S.; Profeta, J.; Grunstein, E. Octreotide treatment of carcinoid hypertensive crisis. Mt. Sinai J. Med. A J. Transl. Pers. Med. 1994, 61, 349–355.
- Veall, M.G.R.Q.; Peacock, M.J.E.; Bax, M.N.D.S.; Reilly, M.C.S. Review of the anaesthetic management of 21 patients under-going laparotomy for carcinoid syndrome. Br. J. Anaesth. 1994, 72, 335–341.
- Wonn, S.M.; Pommier, R.F. Carcinoid crisis: History, dogmas, and data. In Neuroendocrine Tumors; Springer: Berlin, Germany, 2021; pp. 87–103.
- Kinney, M.A.O.; Warner, M.E.; Nagorney, D.M.; Rubin, J.; Schroeder, D.R.; Maxson, P.M. Perianaesthetic risks and outcomes of abdominal surgery for metastatic carcinoid tumours †. Br. J. Anaesth. 2001, 87, 447–452.
- Massimino, K.; Harrskog, O.; Pommier, S.; Pommier, R. Octreotide LAR and bolus octreotide are insufficient for preventing intraoperative complications in carcinoid patients. J. Surg. Oncol. 2013, 107, 842–846.
- Woltering, E.A.; Wright, A.E.; Stevens, M.A.; Wang, Y.-Z.; Boudreaux, J.P.; Mamikunian, G.; Riopelle, J.M.; Kaye, A.D. Development of effective prophylaxis against intraoperative carcinoid crisis. J. Clin. Anesth. 2016, 32, 189–193.
- Condron, M.E.; Pommier, S.J.; Pommier, R.F. Continuous infusion of octreotide combined with perioperative octreotide bolus does not prevent intraoperative carcinoid crisis. Surgery 2016, 159, 358–367.
- Kinney, M.A.; Nagorney, D.M.; Clark, D.F.; O’Brien, T.D.; Turner, J.D.; Marienau, M.E.; Schroeder, D.R.; Martin, D.P. Partial hepatic resections for metastatic neuroendocrine tumors: Perioperative outcomes. J. Clin. Anesth. 2018, 51, 93–96.
- Kwon, D.H.; Paciorek, A.; Mulvey, C.K.; Chan, H.; Fidelman, N.; Meng, L.; Nakakura, E.K.; Zhang, L.; Bergsland, E.K.; Van Loon, K. Periprocedural management of patients undergoing liver resection or embolotherapy for neuroendocrine tumor me-tastases. Pancreas 2019, 48, 496–503.
- Condron, M.E.; Jameson, N.E.; Limbach, K.E.; Bingham, A.E.; Sera, V.A.; Anderson, R.B.; Schenning, K.; Yockelson, S.; Ha-rukuni, I.; Kahl, E.A.; et al. A prospective study of the pathophysiology of carcinoid crisis. Surgery 2018, 165, 158–165.
- Kaltsas, G.; Caplin, M.; Davies, P.; Ferone, D.; Garcia-Carbonero, R.; Grozinsky-Glasberg, S.; Hörsch, D.; Janson, E.T.; Kian-manesh, R.; Kos-Kudła, B.; et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Pre- and perioperative therapy in patients with neuroendocrine tumors. Neuroendocrinology 2017, 105, 245–254.
- Del Olmo-García, M.I.; Muros, M.A.; López-De-La-Torre, M.; Agudelo, M.; Bello, P.; Soriano, J.M.; Merino-Torres, J.-F. Pre-vention and management of hormonal crisis during theragnosis with LU-DOTA-TATE in neuroendocrine tumors. A system-atic review and approach proposal. J. Clin. Med. 2020, 9, 2203.
- de Keizer, B.; Van Aken, M.; Feelders, R.A.; De Herder, W.W.; Kam, B.L.R.; Van Essen, M.; Krenning, E.; Kwekkeboom, D. Hormonal crises following receptor radionuclide therapy with the radiolabeled somatostatin analogue octreotate. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 749–755.
- Stenzel, J.; Noe, S.; Holzapfel, K.; Erlmeier, F.; Eyer, F. Fatal systemic vasoconstriction in a case of metastatic small-intestinal NET. Rep. Gastrointest. Med. 2017, 2017, 9810194.
- Rico, G.T.; Li, M.; Pavlakis, N.; Cehic, G.; Price, T.J. Prevention and management of carcinoid crises in patients with high-risk neuroendocrine tumours undergoing peptide receptor radionuclide therapy (PRRT): Literature review and case series from two Australian tertiary medical institutions. Cancer Treat. Rev. 2018, 66, 1–6.
- Kahil, M.E.; Brown, H.; Fred, H.L. The carcinoid crisis. Arch. Intern. Med. 1964, 114, 26–28.
- Harris, A.L.; Smith, I.E. Tryptophan in the treatment of carcinoid crisis. Cancer Chemother. Pharmacol. 1983, 10, 137–139.
- Hughes, E.W.; Hodkinson, B.P. Carcinoid syndrome: The combined use of ketanserin and octreotide in the management of an acute crisis during anaesthesia. Anaesth. Intensiv. Care 1989, 17, 367–370.
- Mason, D.T.; Melmon, K.L. New understanding of the mechanism of the carcinoid flush. Ann. Intern. Med. 1966, 65, 1334–1339.
- Limbach, K.E.; Condron, M.E.; Bingham, A.E.; Pommier, S.J.; Pommier, R.F. Β-Adrenergic agonist administration is not asso-ciated with secondary carcinoid crisis in patients with carcinoid tumor. Am. J. Surg. 2019, 217, 932–936.
